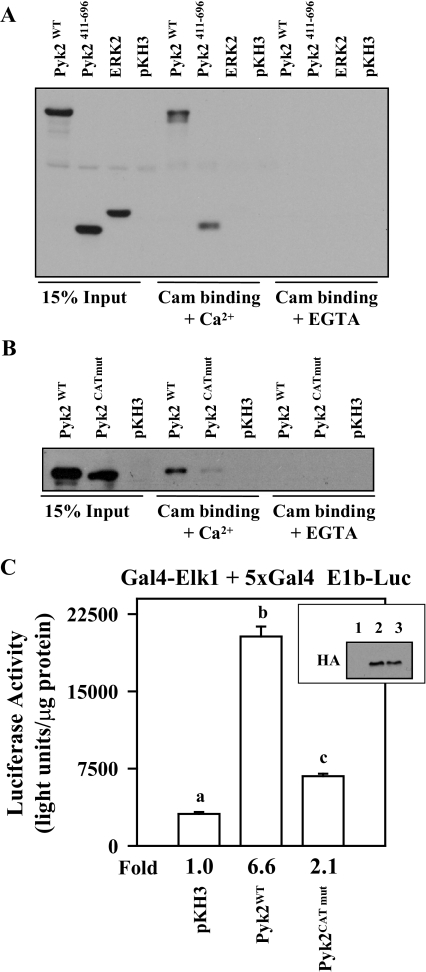
Figure 8.
Mutations within the Putative Cam Binding Site in the Pyk2 Catalytic Domain Block Cam Binding and Reduce Pyk2 Activity
αT3-1 cells were transfected with expression vectors (2 μg each) for HA-tagged wild-type Pyk2 (Pyk2WT), the Pyk2 catalytic domain (Pyk2411–696), full-length HA-tagged ERK2, or the pKH3 parent vector. Twenty-four hours after transfection, cells were lysed and clarified by centrifugation. A portion of the lysate was used for input (15%), and the remainder was split into two binding reactions with Cam agarose in the presence (+Ca2+) or absence (+EGTA) of calcium. A, The binding reactions were washed, resolved using SDS-PAGE, and Western blotted using the HA antibody. B, A similar overexpression study was carried out in αT3-1 cells using wild-type Pyk2 (Pyk2WT) and full-length Pyk2 with the mutation within the putative Cam binding site (Pyk2CATmut). C, The Gal4-Elk1 + 5xGal4-E1b Luc reporter system (500 ng of each plasmid) was transfected into αT3-1 cells with either pKH3 parent vector (5 μg), wild-type Pyk2 (Pyk2WT; 5 μg) or Pyk2 with the mutation within the putative Cam binding site (Pyk2CATmut; 5 μg). Twenty-four hours later, cells were lysed, and luciferase activity was determined. Aliquots from the transfected and lysed cells were subjected to immunoblot analysis using the HA antibody to determine the expression level of the wild-type and mutant Pyk2 proteins (inset: lane 1, pKH3 transfection; lane 2, Pyk2WT; lane 3, Pyk2CATmut).