Abstract
Context:
Whether muscle warming protects against exercise-induced muscle damage is unknown.
Objective:
To determine the effect of leg immersion in warm water before stretch-shortening exercise on the time course of indirect markers of exercise-induced muscle damage.
Design:
Crossover trial.
Setting:
Human kinetics laboratory.
Patients or Other Participants:
Eleven healthy, untrained men (age = 21.5 ± 1.7 years).
Intervention(s):
Participants' legs were immersed in a water bath at 44 ± 1°C for 45 minutes.
Main Outcome Measure(s):
Creatine kinase changes in the blood, muscle soreness, prolonged (within 72 hours) impairment in maximal voluntary contraction force and height of drop jump, and electrically evoked muscle force at low and high stimulation frequencies at short and long muscle lengths.
Results:
Leg immersion in warm water before stretch-shortening exercise reduced most of the indirect markers of exercise-induced muscle damage, including creatine kinase activity in the blood, muscle soreness, maximal voluntary contraction force, and jump height. The values for maximal voluntary contraction force and jump height, however, were higher during prewarming than for the control condition at 48 hours after stretch-shortening exercise, but this difference was only minor at other time points. Muscle prewarming did not bring about any changes in the dynamics of low-frequency fatigue, registered at either short or long muscle length, within 72 hours of stretch-shortening exercise.
Conclusions:
Leg immersion in warm water before stretch-shortening exercise reduced most of the indirect markers of exercise-induced muscle damage. However, the clinical application of muscle prewarming may be limited, because decreasing muscle damage did not necessarily lead to improved voluntary performance.
Keywords: electric stimulation, muscle length, neuromuscular performance, time course
Key Points.
Muscle prewarming did not change maximal voluntary contraction force or jump height during stretch-shortening exercise, but it did increase the ratio of electrically induced muscle force at 100 Hz of long muscle length to short muscle length.
Muscle prewarming affected indirect markers of muscle damage, decreasing blood creatine kinase activity and muscle soreness and increasing maximal voluntary contraction force and jump height within 72 hours after stretch-shortening exercise.
Muscle prewarming did not change the dynamics of low-frequency fatigue in short or long muscle lengths within 72 hours of stretch-shortening exercise.
Exercise-induced muscle damage (EIMD) in humans frequently occurs after unaccustomed exercise, particularly if the exercise involves a large number of eccentric contractions.1–3 The well-documented markers of EIMD include disruption of muscle intracellular structures, sarcolemma, and extracellular matrix2; prolonged impairment of muscle function measured during both voluntary and electrically stimulated contractions1,3; manifestation of low-frequency fatigue (LFF)3,4; creatine kinase (CK) activity in the blood; an acute inflammatory reaction; and delayed-onset muscle soreness, stiffness, and swelling.5,6 One of the indicators of muscle damage evident immediately after eccentric exercise is a shift in the direction of longer muscle lengths in the muscle length-to-tension relationship.2
Warm-up exercise can reduce the extent of EIMD.7,8 Increasing muscle temperature via warm-up exercise could, by enhancing muscle and connective tissue extensibility, decrease EIMD.7 More recently, passive warming before eccentric exercise using pulsed short-wave diathermy increased muscle temperature by approximately 1°C and attenuated swelling but not other clinical markers of muscle damage, including muscle soreness.8 Although passive warming of the forearm flexors pre-exercise was of no benefit in attenuating indicators of muscle damage,9,10 preconditioning with muscle hyperthermia 1 day before eccentric exercise was beneficial.11
Based on these previous studies, the beneficial effect of muscle prewarming in protecting against EIMD is uncertain. This conflict may be due to several causes, namely the protocols used for muscle prewarming, the choice and timing of indirect markers of EIMD, and the characteristics of the exercise used to induce muscle damage. Our aim was to establish the effect of leg immersion in warm water before stretch-shortening exercise (SSE) on indirect markers of EIMD within 72 hours. Because an increase in muscle temperature may increase extensibility of the musculotendinous unit, we anticipated that passive muscle warming before SSE would decrease the manifestations of indirect markers of EIMD.
Methods
Participants
Eleven healthy, untrained men (age = 21.5 ± 1.7 years, height = 179.7 ± 3.5 cm, body mass = 74.2 ± 4.7 kg) took part in this study. Volunteers were physically active (performing moderate-intensity physical activity for 30 minutes or more at least 5 days a week) but did not take part in any sports program and had not been involved in any jumping or leg strength training programs in recent years. Each participant read and signed a written informed consent consistent with the principles outlined in the Declaration of Helsinki. The Ethics Committee of Kaunas Medical University approved this study.
Rationale of Experimental Approach
To examine whether leg immersion in warm water reduced the manifestation of indirect markers of EIMD, each participant performed SSE in 2 conditions in random order: without muscle prewarming (control [C] condition) and with muscle prewarming (W condition). We selected the following indirect markers of EIMD for study: CK activity, muscle soreness, prolonged (within 72 hours) impairment in maximal voluntary contraction force (MVCF) and jump height, and electrically evoked muscle force at low and high stimulation frequencies in short and long muscle lengths. The time interval between exercising in the C and W conditions was 3 to 5 months.
Stretch-Shortening Exercise
The SSE consisted of intermittent drop jumps from a platform (0.5 m high) with countermovement to about a 90° angle at the knees, immediately followed by a maximal vertical jump. Each volunteer performed 3 bouts of 10, 40, and 50 jumps, for a total of 100 jumps per bout. Between jumps, a 30-second rest period was provided, with 5 minutes of rest between bouts. The drop jumps were performed using a multicomponent force plate (model 9286A; Kistler Instrument Corp, Amherst, NY). Jump height (cm) was calculated by applying the following formula: H = 1.226 × Tf2, where Tf = flight time (seconds).12 Participants' hands were positioned on their waists to eliminate arm swing. To avoid additional stress on the right (test) leg, the volunteer stepped on the platform with the left leg (the leg in which muscle contraction force was not measured). After each jump, the participant was informed of his jump height and encouraged to jump as high as possible next time, similar to a protocol applied in previous research.3
Muscle Force Measurements
Equipment and procedures for measuring muscle force were the same as those used in earlier studies.3,13 Participants sat upright in an experimental chair with vertical back support. A strap secured the hips and thighs to minimize uncontrolled movements. The right leg was strapped in a force-measuring device with the knee held at a 90° angle (full leg extension = 180°) for MVCF measurements. A 6-cm-wide plastic cuff, placed around the right leg just proximal to the malleoli, was tightly attached to a linear variable differential transducer. Transducer output, proportional to isometric knee extension force, was amplified and digitized at a sampling rate of 1 kHz by a 12-bit analogue-to-digital converter installed in a personal computer. The digitized signal was stored on a hard disk for subsequent analysis. Force transducer output was also displayed on a voltmeter in front of the volunteer. The MVCF peak was reached and maintained for 2 to 3 seconds before relaxation. The rest interval between MVCF measures was 1 minute.
Equipment and procedures for electric stimulation were essentially the same as described previously.3,13 A high-voltage stimulator (model MG 440; Medicor, Budapest, Hungary) was used. Electric stimuli to the quadriceps muscle were delivered through surface electrodes (9 × 18 cm) padded with cotton cloth and soaked in saline solution. One stimulation electrode was placed just above the patella, while the other covered a large portion of the muscle belly in the proximal third of the thigh. The electrode landmarks were marked with felt-tip pen. Electric stimulation was always delivered in trains of square-wave pulses of 1 millisecond in duration (150 V). With the aim of recruiting the greatest number of fibers, we chose the highest stimulation voltage possible. Before the test stimulation, the muscle was stimulated 2 to 3 times with a single stimulus at 70 to 90 V for familiarization purposes.
The following data were measured: quadriceps muscle after electric stimulation at 20-Hz (P20) and 100-Hz (P100) frequencies. Each electric stimulation series lasted 1 second. We used a force-measuring device to assess the contractile force induced by electric stimulation with the knee kept at an angle of 135° (short muscle length) or 90° (long muscle length) in a randomized manner for each participant. The rest interval between muscle stimulations was 10 seconds. The change in the P20∶P100 ratio after the SSE was used to evaluate low-frequency fatigue.3 The change in the long∶short muscle length ratio with P100 was determined during and after SSE; an increase in this ratio served as an indirect indication of muscle damage.2
Plasma Creatine Kinase Activity
Approximately 5 mL of blood was drawn from the cubital vein into a tube containing lithium heparin before SSE and at 24 and 48 hours after SSE. Blood was centrifuged at 3000 revolutions per minute for 10 minutes, and the separated plasma was removed and stored in a −20°C freezer. Plasma creatine kinase (IU/L−1) activity was determined by using an automatic biochemical analyzer (model Monarch; Instrumentation Laboratory, Giugno, Italy). The normal CK reference range for men with this method is between 24 and 195 IU·L−1.
Muscle Soreness
Muscle soreness was reported subjectively using a visual analogue scale from 0 to 10 points: 0 (none), 1 (very slight), 2 (slight), 3 (mild), 4 (less than moderate), 5 (moderate), 6 (more than moderate), 7 (intense), 8 (very intense), 9 (barely tolerable), and 10 (intolerably intense). Participants were asked to rate the severity of quadriceps soreness when standing up and walking. Muscle soreness was determined at 24, 48, and 72 hours after SSE. A similar method of evaluating muscle soreness has been used by previous researchers.3,14,15
Temperature
We measured muscle temperature by needle thermocouple (model DM-852; Ellab A/S, Roedovre, Denmark) inserted to a depth of 3 cm below the skin surface in the vastus lateralis muscle midthigh and slightly lateral to the femur. The temperature recorded at a 3-cm depth was assumed to represent the average temperature of the active muscle mass.16 We measured rectal temperature using a rectal thermocouple (Ellab A/S, Hvidovre, Denmark) inserted to a depth of 12 cm past the anal sphincter. Room temperature was 22.5°C ± 0.4°C.
Thermal Comfort
We asked participants to rate their thermal comfort using the scale from Gagge et al17 and ranging from 1 (comfortable) to 5 (very uncomfortable). Participants were required to rate their thermal comfort every 5 minutes during leg immersion in warm water and immediately after SSE. Similar methods of thermal comfort have been used in previous research.18,19
Degree of Dehydratation
The volunteers, naked and having wiped themselves dry, were weighed on an electronic scale (model TBF 300; Tanita, Tokyo, Japan) before and after passive muscle prewarming. The difference in weight reflected the amount of fluid lost by the participant during leg immersion in warm water. Volunteers were not allowed to urinate or consume liquids between weight measurements.
Experimental Protocol
The experimental design with muscle prewarming is shown in Figure 1. After we measured CK activity and baseline temperature (muscle and rectal), the participant was seated in the experimental chair, and muscle contractile properties were recorded in the following sequence: P20, P100 and MVCF (MVCF was reached 3 times, with the highest value used for evaluation). Then the participant warmed up by running in place for 5 minutes with an intensity corresponding to a heart rate of 130 to 150 beats per minute (approximately 70% of maximum heart rate) and 10 squat-stands. Heart rate was measured with a Polar recorder (model S625X; Polar Electro, Kempele, Finland).
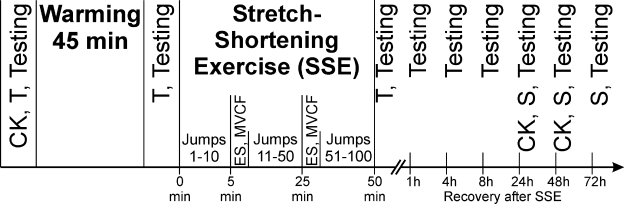
Figure 1. The experimental design with muscle pre-warming. CK indicates creatine kinase activity measurement; T, rectal and muscle temperature measurements; ES, electric stimulation of quadriceps femoris muscle with different frequencies at knee joint angles of 135° and 90° in a randomized manner for each participant; MVCF, maximal voluntary contraction force measurement at the 90° knee angle; S, soreness measurement; Testing, ES, jump height, and MVCF measurement.
Then control jump height was established. Each volunteer performed 3 to 5 jumps every 30 seconds, and the best attempt was selected. Techniques and equipment used for control jumps were the same as described in the section on SSE. Participants in the W condition sat in a 44°C water bath for 45 minutes in waist-high water. Thermal comfort was recorded every 5 minutes during warming. Immediately after muscle warming, the needle thermocouple was inserted and rectal temperature measured. Control jump height, P20, P100, and MVCF were measured 1 to 3 minutes after muscle warming ended. Then participants in condition W performed SSE (100 intermittent drop jumps). After 10, 50, and 100 drop jumps, the volunteers were again seated in the experimental chair for measurement of voluntary (2 MVCF attempts) and electrically induced muscle contractions. Immediately after SSE, muscle temperature, rectal temperature, and thermal comfort were recorded. At 1, 4, 8, 24, 48, and 72 hours after SSE, voluntary and electrically induced muscle contractions and control jump height were recorded in the same sequence as before the load was applied. Muscle soreness was determined at 24, 48, and 72 hours after SSE. Creatine kinase activity was measured at 24 and 48 hours after SSE.
The experimental design without muscle prewarming was the same except that the participants performed SSE immediately after warm-up and pre-exercise measurements.
Data and Statistical Analysis
A 2-way repeated-measures analysis of variance was used to determine the effect of time (10 levels: before and after 10, 50, and 100 drop jumps as well as during recovery within 1, 4, 8, 24, 48, and 72 hours) and temperature (2 levels: with or without passive warming) on voluntary and electrically evoked muscle performance. When effects were found, we conducted paired comparisons between measurements with a least significant difference correction. The level of significance was set at .05.
Results
Testing Muscle Function Before Stretch-Shortening Exercise
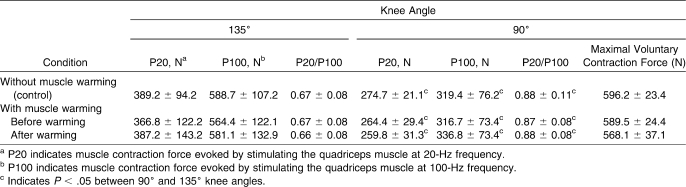
We found no difference between the C and W conditions in pre-exercise values of MVCF and muscle contraction force evoked at different electric stimulation frequencies (P > .05, Table 1). Still, in both conditions, muscle force evoked by electric stimulation at 20 Hz and 100 Hz frequencies at short muscle length was greater than at long muscle length (LL) (P < .01, power > 99%).
Table 1.
Pre-Exercise Values of Voluntary and Electric Stimulation-Induced Quadriceps Muscle Contractions (Mean ± SD)
Thermoregulatory Data and Thermal Comfort
Differences between pre-immersion and postimmersion were seen in both rectal (37.3°C ± 0.32°C and 39.1°C ± 0.31°C, respectively; P < .01, power = 99%) and muscle (36.7°C ± 0.3°C and 39.7°C ± 0.3°C, respectively; P < .001, power = 99%) temperature in the W condition. Immediately after SSE in the C and W conditions, rectal temperature was 37.8°C ± 0.4°C and 38.9°C ± 0.3°C, respectively (P < .05, power = 95%), and muscle temperature was 37.5°C ± 0.4°C and 39.4°C ± 0.4°C, respectively (P < .05, power = 95%).
After leg immersion in warm water, participants rated thermal comfort at 4.2 ± 0.5 points on the Gagge et al17 scale. Immediately after SSE, thermal comfort in the C and W conditions decreased to 1.2 ± 0.1 and 3.2 ± 0.4 points, respectively (P < .05, power = 95%).
During leg immersion in warm water, participants lost 0.93 ± 0.3 kg, which constituted 1.17% ± 0.4% of their body mass. This change was not significant (P > .05).
Changes in Voluntary Muscle Performance
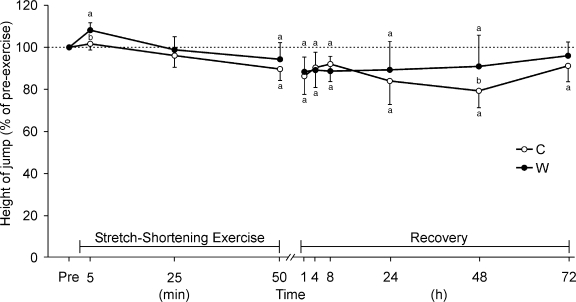
In the C condition, jump height before SSE was 40.2 ± 7.2 cm. In the W condition, jump height before warming was 40.9 ± 8.7 cm (P > .05). After warming, jump height was 44.3 ± 8.1 cm (postwarming versus prewarming: P < .05, power = 24%).
The time courses of jump height and MVCF during and after SSE in both the C and W conditions are shown in Figures 2 and 3 (time effect: P < .001, temperature effect: P < .001). In neither condition did MVCF recover to pre-exercise level within 72 hours (C condition: P < .05, power = 49%; W condition: P < .05, power = 45%). Secondary decreases were noted in jump height and MVCF from 8 to 48 hours after SSE in the C condition but not in the W condition (P > .05). Jump height and MVCF, however, were different between the C and W conditions at 48 hours after SSE (P < 0.05, power > 26%, and P < .05, power > 95%, respectively).
Figure 2. Time course of changes in jump height during and after stretch-shortening exercise of 100 drop jumps every 30 seconds. Measurements were taken after 10 (minute 5), 50 (minute 25), and 100 (minute 50) drop jumps and during recovery at 1, 4, 8, 24, 48, and 72 hours after performing 100 jumps. C indicates control condition without muscle prewarming; W, condition with muscle prewarming. a Indicates P < .05 between pre-exercise (Pre) and postexercise levels; b P < .05 between C and W conditions.
Figure 3. Time course of changes in maximal voluntary contraction force (MVCF) during and after stretch-shortening exercise of 100 drop jumps every 30 seconds. Measurements were taken after 10 (minute 5), 50 (minute 25), and 100 (minute 50) drop jumps and during recovery at 1, 4, 8, 24, 48, and 72 hours after performing 100 jumps. C indicates control condition without muscle prewarming; W, condition with muscle prewarming. a Indicates P < .05 between pre-exercise (Pre) and postexercise levels; b P < .05 between C and W conditions.
Changes in Electrically Induced Muscle Performance
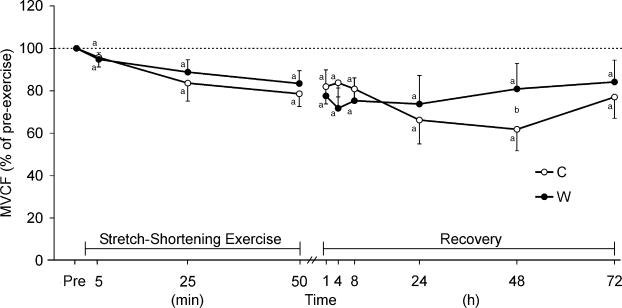
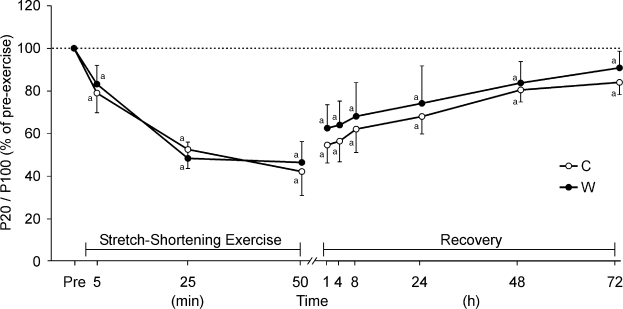
Muscle contraction force induced by different electric stimulation frequencies decreased immediately after SSE. In addition, muscle force induced by low frequencies and indicative of low-frequency fatigue decreased. Immediately after SSE, low-frequency fatigue manifested more markedly in short muscle lengths than in long muscle lengths (P < .05, power = 87%; Figure 4). No difference was observed in low-frequency fatigue registered during SSE and throughout recovery in condition C or W.
Figure 4. Time course of changes in P20/P100 ratio at short muscle length during and after stretch-shortening exercise of 100 drop jumps every 30 seconds. Measurements were taken after 10 (minute 5), 50 (minute 25), and 100 (minute 50) drop jumps and during recovery at 1, 4, 8, 24, 48, and 72 hours after performing 100 jumps. C indicates control condition without muscle prewarming; W, condition with muscle prewarming. a Indicates P < .05 between pre-exercise (Pre) and postexercise levels.
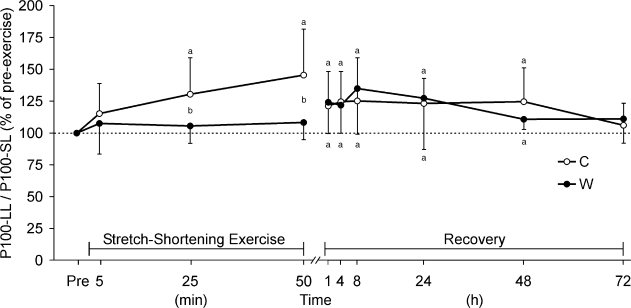
Due to greater P100 reduction at short muscle lengths, the ratio of P100 at long lengths to P100 at short lengths increased immediately after SSE in the C condition (P < .01, power > 78%; Figure 5). The ratio from 1 to 48 hours remained higher than the initial ratio (P < .05, power > 59%). No differences were apparent, however, between the C and W conditions from 1 to 72 hours after SSE (P > .05).
Figure 5. Time course of changes in the ratio of P100 (muscle contraction force evoked by stimulating the quadriceps muscle at 100-Hz frequency) at long to short muscle length during and after stretch-shortening exercise of 100 drop jumps every 30 seconds. Measurements were taken after 10 (minute 5), 50 (minute 25), and 100 (minute 50) drop jumps and during recovery at 1, 4, 8, 24, 48, and 72 hours after performing 100 jumps. C indicates control condition without muscle prewarming; W, condition with muscle prewarming. a Indicates P < .05 between pre-exercise (Pre) and postexercise levels; b P < .05 between conditions C and W.
Creatine Kinase and Muscle Soreness
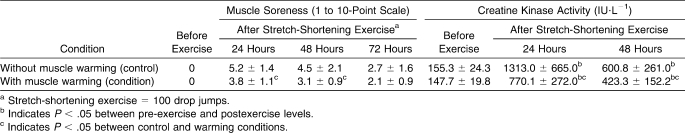
The CK activity in blood registered at 24 and 48 hours after SSE was considerably smaller in the W than the C condition (P < .05, power > 91%; Table 2). A similar effect of leg immersion in warm water on muscle soreness was observed at 24 and 48 hours after SSE, although it was smaller in the W condition than in the C condition (P < .05, power from 60% to 21%).
Table 2.
Muscle Soreness and Creatine Kinase Activity Before and After Stretch-Shortening Exercise (Mean ± SD)
Discussion
Our experiments were designed to test the hypothesis that muscle warming before SSE reduces EIMD. Our main findings are as follows: 1) muscle prewarming did not cause any changes in jump height or MVCF during SSE, but prewarming decreased the ratio of electrically induced muscle force at 100 Hz at long versus short muscle lengths; 2) muscle prewarming affected indirect markers of muscle damage (decreasing CK activity in blood and muscle soreness and increasing MVCF and jump height) within 72 hours after SSE; and 3) muscle prewarming did not bring about any changes in the dynamics of low-frequency fatigue registered at short or long muscle lengths within 72 hours after SSE.
To our knowledge, this is the first study devoted to analyzing the effect of passive muscle prewarming via the most frequently used indirect markers of muscle damage (CK activity, muscle soreness, and prolonged impairment in voluntary muscle performance) and to evaluating the prolonged impairment of electrically evoked muscle force at low and high frequencies in short and long muscle lengths.
The main causes of changes in voluntary and electrically induced muscle performance are related to EIMD. Our results showed that passive muscle prewarming increased jump height but did not bring about any changes in MVCF, P20, or P100 in short or long muscle lengths. Davies and Young20 demonstrated that a mean muscle temperature rise of 3.1°C was associated with decreases in time to peak tension and half-relaxation time, but does not affect tetanic tension at 20 Hz or 40 Hz or maximal voluntary contraction force. The increase in jump height is in accord with other findings16 that muscle contraction capacity increases as muscle temperature increases. Thus, after leg immersion in a warm water protocol analogous to ours, Sargeant16 noted muscle temperature of 39.3°C at a 3-cm depth and increases in peak power on an isokinetic cycle ergometer (approximately 11%) and fatigue rate.
Changes in both voluntary and electrically evoked muscle performance during SSE are not likely attributable to an increase in the myoplasm of metabolites, such as phosphate and hydrogen ions, because the jump duration (0.5 to 0.55 seconds) was too short for adenosine triphosphate (ATP) and phosphocreatine to decrease, whereas the resting period of 30 seconds was sufficient for ATP and phosphocreatine to be restored.21 The causes of changes in muscle performance when performing SSE are, therefore, associated with nonmetabolic factors, most likely related to muscle damage.
After performing SSE in both the C and W conditions, indirect markers of muscle damage manifested within 24 to 72 hours after the load with rises in muscle soreness and CK activity (Table 2), and prolonged impairment of neuromuscular performance (jump height and MVCF; Figures 2 and 3). The main reasons for the decrease in jump height and MVCF and increase in low-frequency fatigue were likely related to damage to the force-bearing structures and the excitation-contraction coupling system.2,22
Our results suggest that reductions in electrically evoked muscle force after SSE depend on the muscle length at which they were measured. Previous researchers22 studying the knee extensors also found strength loss after exercise depended on length. However, they measured voluntary muscle force.
Fatigue increases with hyperthermia and may be caused by a decline in voluntary activation of muscle (ie, central fatigue).18,23–26 During passive elevation of core temperature to approximately 39.5°C, voluntary activation of knee extensors decreased during maximal voluntary contractions lasting 10 seconds.18 Therefore, we do not believe that increased rectal temperature in our participants had an essential effect on changes in jump height. As noted by Cheung and Sleivert,23 force and activation are well sustained during brief contractions. Also, Nybo and Nielsen24 observed no initial decrease in voluntary activation after exercise-induced hyperthermia, but activation decreased when the contraction was maintained over the subsequent 120 seconds.
Muscle Warming Before Stretch-Shortening Exercise Reduced Main Markers of Exercise-Induced Muscle Damage
Leg immersion in warm water before SSE affected the indirect markers of EIMD by reducing CK activity in the blood and muscle soreness, and preventing prolonged decreases in jump height and MVCF. The decrease in muscle damage is likely due to muscle prewarming. However, jump height and MVCF values were higher in prewarming than in the C condition 48 hours after SSE. This difference was minor at other time points, leading to the assumption that less muscle damage does not necessarily mean better performance.
Our results did not support the findings of Nosaka et al9 and Brock Symons et al10 that an increase in muscle temperature due to passive warming before eccentric exercise had no effect on changes in maximal isometric force, muscle soreness, or plasma CK activity. Our data showed a prewarming effect in decreasing EIMD. However, differences may be due to variations in experimental protocols, including muscles tested, prewarming treatment, and exercise used to cause EIMD. Nosaka et al9 and Brock Symons et al10 used eccentric exercise, whereas we used intermittent drop jumps with countermovements, immediately followed by maximal vertical jumps.
Our results support conclusions of other authors that passive prewarming is effective in reducing EIMD.27 Although the mechanisms of this phenomenon are unclear, passive prewarming may increase the extensibility of the musculotendinous unit, decrease muscle viscosity and smooth contractions, thereby reducing susceptibility to strain injury. All these factors may have reduced muscle stress in the W condition.
Another possible explanation requiring further study is the release of heat shock proteins. Hyperthermia or exercise increases heat shock proteins in skeletal muscle, which may protect the muscle from further damage.28 Unfortunately, we were not able to analyze heat shock proteins in our study.
It is rather unusual that prewarming caused no changes in the time course of muscle force induced by different stimulation frequencies at different muscle lengths during and after SSE. However, muscle prewarming resulted in a decrease in the long to short muscle length ratio of P 100 during SSE (Figure 5). This finding reflects the damage muscles were subjected to during SSE. One indicator of muscle damage immediately after eccentric exercise is a shift toward longer muscle lengths in the muscle length–tension relationship.2 The popping sarcomere hypothesis of muscle damage suggests that the muscle length-tension relationship undergoes a shift to the right, toward longer muscle length, after eccentric exercise.2 Still, it is rather strange that this phenomenon disappeared soon after SSE (Figure 5).
Our results showed that prewarming had no effect on the dynamics of low-frequency fatigue registered at either long or short muscle lengths during and within 72 hours after SSE (Figure 4). We have not identified any research devoted to the effect of prewarming on the dynamics of low-frequency fatigue, which is characterized by a relative loss of force at low frequencies; it is important to mention that the force is not impaired or is only mildly impaired at high frequencies.13,29 Although the underlying mechanism is unknown, metabolite build-up, elevation of intracellular calcium, and mechanical damage to the muscle have been suggested to play a role in the development of low-frequency fatigue.29
Conclusions
Leg immersion in warm water before SSE decreased the manifestation of indirect markers of muscle damage during, immediately after, and within 72 hours after SSE. Still, muscle prewarming did not cause any changes in the dynamics of low-frequency fatigue for either long or short muscle lengths. The clinical application of muscle prewarming is limited, as reducing the damage does not necessarily lead to improved voluntary performance.
Footnotes
Albertas Skurvydas, PhD, contributed to conception and design; analysis and interpretation of the data and drafting and final approval of the paper. Sigitas Kamandulis, PhD, contributed to acquisition and analysis and interpretation of the data and drafting and final approval of the paper. Aleksas Stanislovaitis, PhD, and Vytautas Streckis, PhD, contributed to acquisition of the data and drafting, critical revision, and final approval of the paper. Gediminas Mamkus, PhD, and Adomas Drazdauskas, PhD, contributed to analysis and interpretation of the data and critical revision and final approval of the paper.
References
- 1.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Proske U, Allen T.J. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33(2):98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Skurvydas A, Sipaviciene S, Krutulyte G, et al. Dynamics of indirect symptoms of skeletal muscle damage after stretch-shortening exercise. J Electromyogr Kinesiol. 2006;16(6):629–636. doi: 10.1016/j.jelekin.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Komi P.V. Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J Biomech. 2000;33(10):1197–1206. doi: 10.1016/s0021-9290(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson P.M, Hubal M.J. Exercise-induced muscle damage in humans. J Physiol Med Rehabil. 2002;81(suppl 11):552–569. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Friden J, Lieber R.L. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand J Med Sci Sports. 2001;11(2):126–127. doi: 10.1034/j.1600-0838.2001.011002126.x. [DOI] [PubMed] [Google Scholar]
- 7.Rodenburg J.B, Steenbeek D, Schiereck P, Bar P.R. Warm-up, stretching and massage diminish harmful effects of eccentric exercise. Int J Sports Med. 1994;15(7):414–419. doi: 10.1055/s-2007-1021080. [DOI] [PubMed] [Google Scholar]
- 8.Evans R.K, Knight K.L, Draper D.O, Parcell A.C. Effects of warm-up before eccentric exercise on indirect markers of muscle damage. Med Sci Sports Exerc. 2002;34(12):1892–1899. doi: 10.1097/00005768-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Nosaka K, Sakamoto K, Newton M, Sacco P. Influence of pre-exercise muscle temperature on responses to eccentric exercise. J Athl Train. 2004;39(2):132–137. [PMC free article] [PubMed] [Google Scholar]
- 10.Brock Symons T, Clasey J.L, Gater D.R, Yates J.W. Effects of deep heat as preventative mechanism on delayed onset muscle soreness. J Strength Cond Res. 2004;18(1):155–161. doi: 10.1519/1533-4287(2004)018<0155:eodhaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Nosaka K, Muthalib A, Lavender A, Laursen P.B. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol. 2007;99(2):183–192. doi: 10.1007/s00421-006-0331-5. [DOI] [PubMed] [Google Scholar]
- 12.Bosco C, Viitasalo J.T, Komi P.V, Luhtanen P. Combined effect of elastic energy and myoelectrical potentiation during stretch-shortening cycle exercise. Acta Physiol Scand. 1982;114(4):557–565. doi: 10.1111/j.1748-1716.1982.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 13.Ratkevicius A, Skurvydas A, Povilonis E, Quistorff B, Lexell J. Effects of contraction duration on low-frequency fatigue in voluntary and electrically induced exercise of quadriceps muscle in humans. Eur J Appl Physiol Occup Physiol. 1998;77(5):462–468. doi: 10.1007/s004210050361. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson P.M, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol. 1988;65(1):1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Allen J.D, Mattacola C.G, Perrin D.H. Effect of microcurrent stimulation on delayed-onset muscle soreness: a double-blind comparison. J Athl Train. 1999;34(4):334–337. [PMC free article] [PubMed] [Google Scholar]
- 16.Sargeant A.J. Effect of muscle temperature on leg extension force and short-term power output in humans. Eur J Appl Physiol Occup Physiol. 1987;56(6):693–698. doi: 10.1007/BF00424812. [DOI] [PubMed] [Google Scholar]
- 17.Gagge A.P, Stolwijk J.A, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res. 1969;2(3):209–229. doi: 10.1016/0013-9351(69)90037-1. [DOI] [PubMed] [Google Scholar]
- 18.Morrison S, Sleivert G.G, Cheung S.S. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol. 2004;91(5–6):729–736. doi: 10.1007/s00421-004-1063-z. [DOI] [PubMed] [Google Scholar]
- 19.Geurts C.L, Sleivert G.G, Cheung S.S. Local cold acclimation during exercise and its effect on neuromuscular function of the hand. Appl Physiol Nutr Metab. 2006;31(6):717–725. doi: 10.1139/h06-076. [DOI] [PubMed] [Google Scholar]
- 20.Davies C.T, Young K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J Appl Physiol. 1983;55(1, pt 1):191–195. doi: 10.1152/jappl.1983.55.1.191. [DOI] [PubMed] [Google Scholar]
- 21.Vollestad N.K, Sejersted O.M, Bahr R, Woods J.J, Bigland-Ritchie B. Motor drive and metabolic responses during repeated submaximal contractions in humans. J Appl Physiol. 1988;64(4):1421–1427. doi: 10.1152/jappl.1988.64.4.1421. [DOI] [PubMed] [Google Scholar]
- 22.Byrne C, Eston R.G, Edwards R.H.T. Characteristics of isometric and dynamic strength loss following eccentric exercise-induced muscle damage. Scand J Med Sci Sports. 2001;11(3):134–140. [PubMed] [Google Scholar]
- 23.Cheung S.S, Sleivert G.G. Multiple triggers for hyperthermic fatigue and exhaustion. Exerc Sport Sci Rev. 2004;32(3):100–106. doi: 10.1097/00003677-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91(3):1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- 25.Todd G, Butler J.E, Taylor J.L, Gandevia S.C. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563(pt 2):621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin P.G, Marino F.E, Rattey J, Kay D, Cannon J. Reduced voluntary activation of human skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp Physiol. 2005;90(2):225–236. doi: 10.1113/expphysiol.2004.028977. [DOI] [PubMed] [Google Scholar]
- 27.Safran M.R, Seaber A.V, Garrett W.E., Jr Warm-up and muscular injury prevention. An update. Sports Med. 1989;8(4):239–249. doi: 10.2165/00007256-198908040-00004. [DOI] [PubMed] [Google Scholar]
- 28.Kregel K.C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92(5):2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 29.Westerblad H, Allen D.G. Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol. 2002;14(6):648–652. doi: 10.1097/00002281-200211000-00003. [DOI] [PubMed] [Google Scholar]