Figure 4. Details of Eaf3 Chromo Barrel Domain Interaction with the Linked Histone H3KC36me2 Sequence.
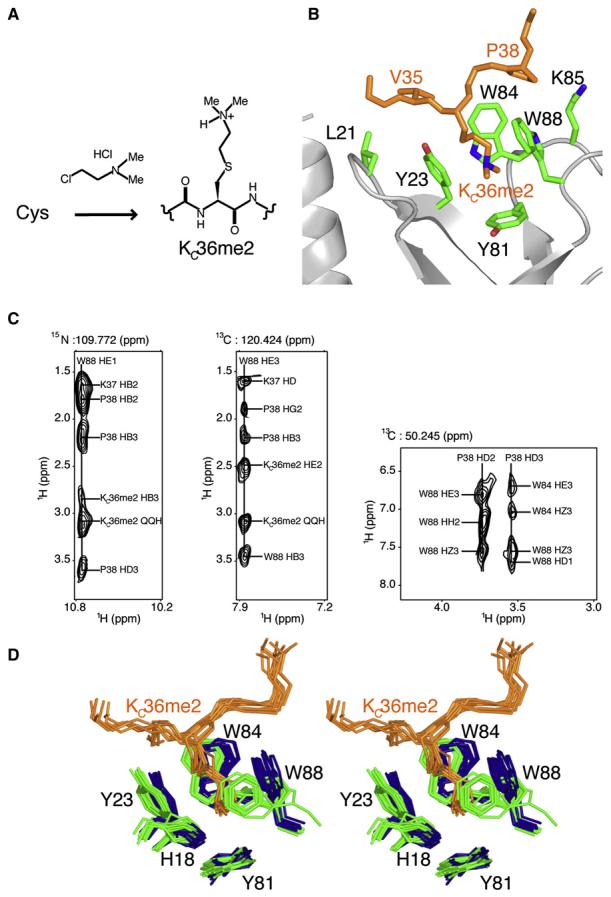
(A) Chemical conversion of a cysteine residue to a dimethyllysine analog (KCme2).
(B) Close-up view of the main interaction site within the Eaf3-H3KC36me2 protein. Tyr23, Tyr81, Trp84, and Trp88 of the chromo barrel domain of Eaf3 form an aromatic cage that accommodates the linked dimethylated lysine analog of H3K36. Other residues (Leu21 and Lys85 of Eaf3; and V35 and Pro38 of linked H3KC36me2) involved in the interaction are also labeled.
(C) Planes from the 3D 15N nuclear Overhauser effect spectroscopy (NOESY) experiment showing NOE correlations of W88HE1 of Eaf3 to KC36me2, Lys37, and Pro38 of linked H3KC36me2 (left); and 13C-edited NOESY experiments showing NOE correlations of W88HE3 of Eaf3 to KC36me2, Lys37, and Pro38 of linked H3KC36me2 (middle), and NOE correlations of the HD protons of P38 of linked H3KC36me2 to the aromatic protons of Trp84 and Trp88 of Eaf3 (right).
(D) Stereo view of the superposition of 10 NMR structures each of free Eaf3 (blue) and Eaf3-H3KC36me2 complex (Eaf3 in green and H3KC36me2 in orange) showing a close-up representation of the aromatic pocket binding site.
