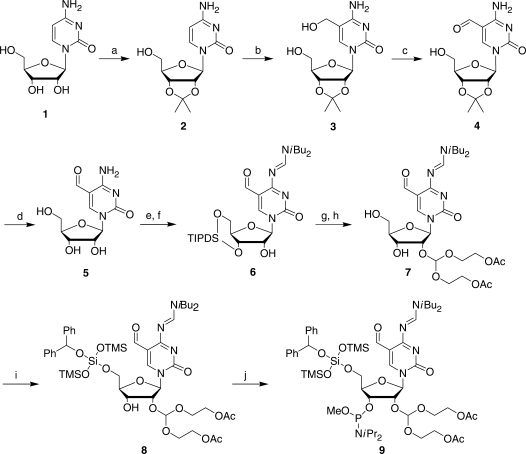
Figure 2.
Synthesis of the 5-formylcytidine phosphoramidite. The starting compound cytidine is numbered compound 1, intermediates are numbered 2–8 and the protected f5C phosphoramidite is compound 9. The synthetic transformations are: (a) acetone, dimethoxypropane, cat HClO4 (83%); (b) paraformaldehyde, 0.5 M KOH, 55°C (38%, 60% brsm); (c) RuO2 xH2O, dioxane, reflux (82%); (d) 1 M HCl (95%); (e) TIPDSCl2, Pyr, DMF; (f) DBF-CH(OMe)2, DMF (71% over 2 steps); (g) ACE-orthoester, PPTS, TBDMS-pentanedione, DCM; (h) HF-TEMED, CH3CN (53% over 2 steps); (i) BZH-Cl, DIA, DCM (84%); (j) P(OMe)(DIA)2, DIA, S-Et Tetrazole, DCM (93%).