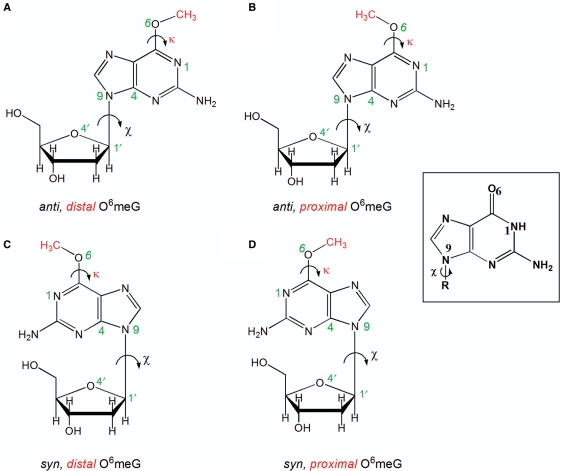
Figure 1.
Structures of the four possible conformations of O6-meG. The glycosidic torsion angle (χ) defined by the atoms O4′–C1′–N9–C4 is shown in black and the torsion angle (κ) defined by the atoms N1–C6–O6–C is displayed in red. The angle χ is shown in both the anti (the six-membered ring points away from the sugar) or syn (the six-membered ring is over the sugar) conformations, while the torsion angle (κ) is in the distal (facing N1–C6) or the proximal (facing away from N1–C6) orientation. These torsional possibilities give rise to four conformational combinations for the lesion (A) anti,distal (B) anti,proximal (C) syn,distal and (D) syn,proximal O6-meG. Guanine is shown as an inset to highlight the different hydrogen-bond donor/acceptor properties.