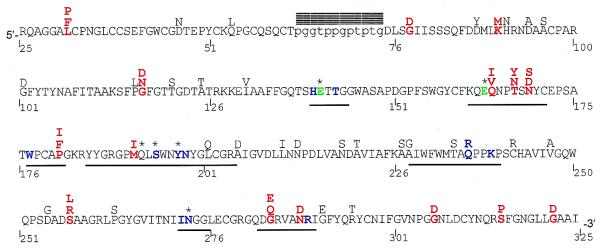
Figure 1.
A. parishii class I chitinase amino acid sequence. Residue number 1 corresponds to the start codon, but only residues 25–325 are included in this study. The mature protein is ≈298 residues long, consisting of a cysteine-rich chitin binding domain (5′) and a chitinolytic domain (3′), connected by a hypervariable proline-glycine rich hinge (lowercase). Residues 1–22 and 319–325 form the signal peptide and vacuolar targeting peptide, respectively, and are cleaved from the mature peptide. Positively selected residues are red, catalytic residues Glu-141 and Glu-163 are green, putative substrate-binding residues are shown in blue (40), active site residues (defined as residues within 0.6 nm of bound substrate) are underlined, blocks denote indels, and * denotes importance for enzyme function confirmed by directed mutagenesis (51, 52). Alternative residues found among the 19 sequences are shown above the A. parishii sequence. Positively selected residues are identified as sites having a posterior probability > 0.95 of being in the positive category, for a majority of phylogenies tested.