Figure 7.
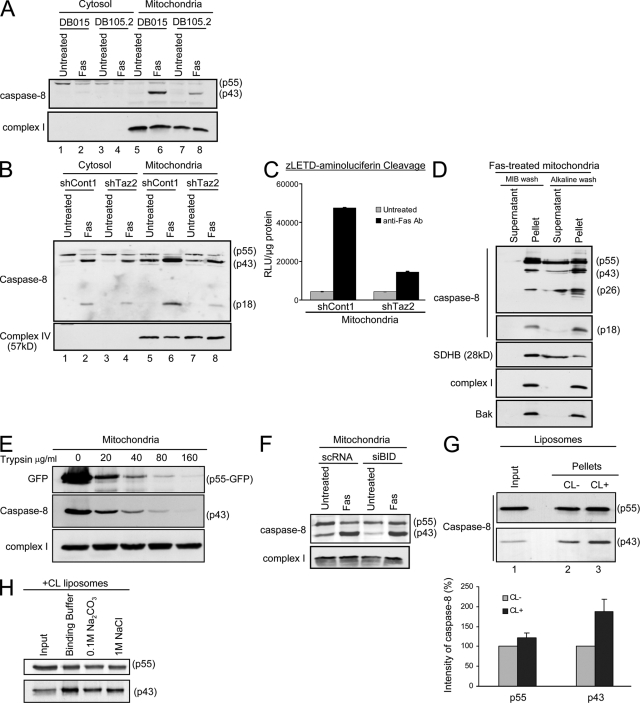
Caspase-8 translocation to the mitochondria is blocked in CL-deficient cells. (A) Control (DB015) and Barth syndrome–derived (DB105.2) lymphoblastoid cells were either left untreated or treated with anti-Fas antibody for 24 h. Cytosolic and mitochondria-enriched fractions were isolated, and caspase-8 localization and autoprocessing was analyzed by Western blotting using the anti-DED antibody. Subunit 6 of complex I was used as a mitochondrial marker and loading control. (B) The different HeLa-derived clones were treated as indicated, and cells were fractionated to cytosolic and mitochondria-enriched fractions. The cleaved products of caspase-8 were analyzed with the anti-p18 antibody. Subunit 4 of complex IV was used as a mitochondrial marker and loading control. (C) Mitochondria were isolated from HeLa cells treated with anti-Fas antibody as indicated. The cleavage of zLETD-aminoluciferin was used to assess caspase-8 activity on the mitochondria. The graph shows the relative levels of emitted signal from the experiment displayed in arbitrary units of luminescence (RLU) per microgram of protein. Error bars represent ± standard deviation. (D) Mitochondria were isolated from HeLa cells treated with anti-Fas antibody and either washed with MIB only or with alkaline MIB. The pellet and supernatant of each wash were analyzed for caspase-8 (DED and p18 domains). The different cleaved products of caspase-8 are indicated on the right. Succinate dehydrogenase complex subunit B (SDHB) was used as a control for loosely attached proteins, and complex I and Bak were used as control for membrane-inserted proteins. (E) Mitochondria were isolated from HeLa cells transfected with caspase-8–C360S–GFP in the presence of zVAD-fmk. Isolated mitochondria were subjected to trypsin digestion at the indicated concentrations. Mitochondrial pellets were analyzed by immunoblotting. (F) HeLa cells were transiently transfected with either control siRNA (scRNA) or siRNA targeting Bid (siBid), as in Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200803129/DC1). 48 h later, cells were either left untreated or treated with anti-Fas antibody for 14 h. Mitochondria were then isolated, and caspase-8 localization and autoprocessing was detected by Western blotting using the anti-DED antibodies. Subunit 6 of complex I was used as loading control. (G) Liposomes were prepared with or without CL and incubated for 30 min at 37°C with in vitro translated procaspase-8 protein. Liposomes were then precipitated and washed, and caspase-8 binding to the lipid membrane was analyzed by Western blotting. The input lane represents 20% of the sample after incubation with caspase-8 but before precipitation. The graph shows the relative intensity of the different caspase-8 forms on liposomes with or without CL, and is the mean and standard deviation of three independent experiments. Error bars represent ± standard deviation. (H) CL-containing liposomes were incubated with caspase-8 and either washed once in binding buffer, or in alkalic (0.1 M Na2CO3, pH 11.5) or high-salt (1 M NaCl) buffers. After precipitation of liposomes, caspase-8 levels were assessed by immunoblotting. The input lane is as in G.