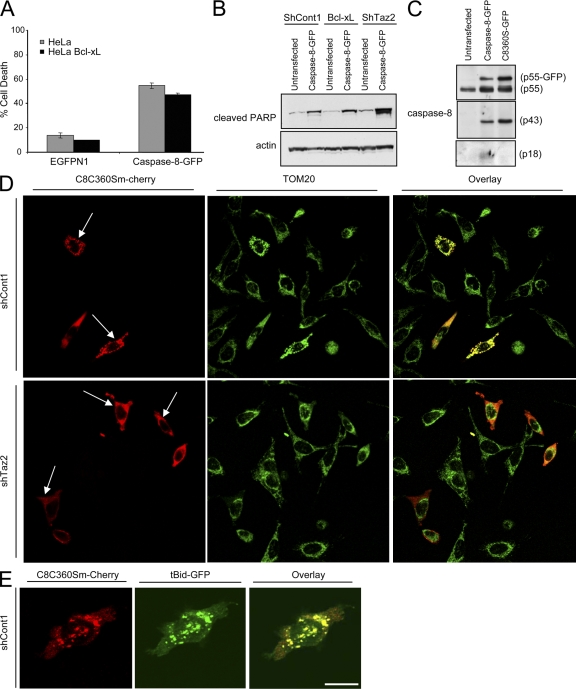
Figure 8.
Caspase-8 C360S colocalizes with tBid on the mitochondria. (A) Parental or Bcl-xL–overexpressing HeLa cells were transfected with either empty vector (EGFPN1) or with a plasmid encoding the caspase-8–GFP fusion protein. Green cells were analyzed by FACS for viability using PI exclusion. Error bars represent ± standard deviation. (B) The indicated HeLa cells were either left untransfected or transfected with the caspase-8–GFP fusion protein. 24 h later, cells were lysed and analyzed for PARP cleavage (using anti-cleaved PARP antibody) as an indicator of cell death. (C) HeLa cells were either left untransfected or transfected with the indicated caspase-8–GFP protein. The presence of the different caspase-8 processed forms was detected using different anti–caspase-8 antibodies. (D) Control and tafazzin-knockdown cells were transfected with caspase-8 C360S mutant fused to m-Cherry (C8C360S–m-Cherry), and 24 h later, cells were fixed and stained for TOM20 as a mitochondrial marker. Alexa 647–conjugated secondary antibody was used for a far-red fluorescence detection of the mitochondria (artificially presented in green). (E) Control HeLa cells were transfected with C8C360S–m-Cherry and tBid-GFP in the presence of zVAD-fmk, and 16 h later, cells were fixed and analyzed by confocal microscopy. Bars, 10 μm.