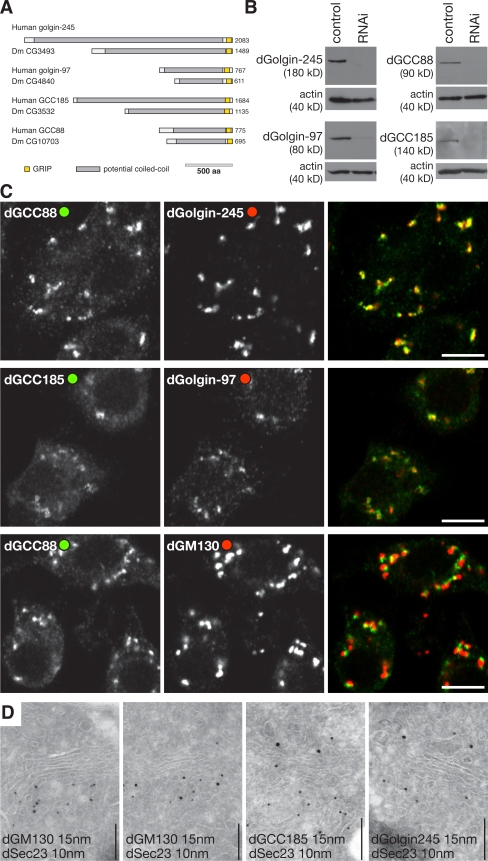
Figure 1.
Drosophila GRIP domain proteins localize to the Golgi apparatus. (A) Schematic representation of the human GRIP domain proteins and their Drosophila orthologues. Colors indicate regions predicted to be predominantly coiled-coil (gray) or the GRIP domains (yellow). (B) Protein blots of S2 lysates probed with antisera against the indicated GRIP domain proteins. The cells had been treated with dsRNA against GFP (control) or the corresponding GRIP domain proteins. Each antibody recognizes a single band, which is strongly reduced after RNAi. (C) Confocal micrographs of S2 cells labeled with the indicated antibodies. dGolgin-245 is coincident with dGCC88, and dGolgin-97 is coincident with dGCC185, but all GRIP domain proteins, here represented by dGCC88, are close to but slightly displaced from the cis-Golgi–specific dGM130. Bars, 5 μm. (D) Immunoelectron micrographs of frozen cryosections of S2 cells double labeled for dSec23 (15-nm gold), a marker of ER exit sites, and the indicated coiled-coil proteins (10-nm gold). dGM130 is present on the cis-Golgi cisternae that are adjacent to exit sites, whereas the GRIP proteins are on the opposite side of the stack. Sectioning and labeling were performed as described previously (Friggi-Grelin et al., 2006). Bars, 200 nm.