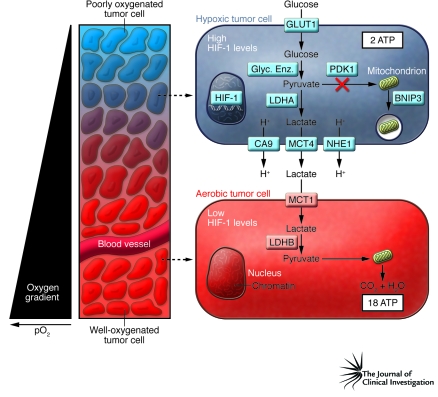
Figure 1. Intratumoral hypoxia and metabolic symbiosis.
Tumors are characterized by gradients of O2 levels, based on the distance of tumor cells from a functional blood vessel. In the schematic, tumor cells surrounding the blood vessel are well oxygenated, whereas the tumor cells more distant from the vessel are poorly oxygenated and express high levels of HIF-1. HIF-1 induces the expression of proteins that increase: uptake of glucose (e.g., glucose transporter 1 [GLUT1]); conversion of glucose to pyruvate (e.g., glycolytic enzymes [Glyc. Enz.]); generation of lactate and H+ (e.g., LDHA); and efflux of these molecules out of the cell (e.g., carbonic anhydrase IX [CA9], sodium-hydrogen exchanger 1 [NHE1], MCT4). Two moles of lactate are produced for each mole of glucose consumed by the hypoxic cell. This increase in glycolytic metabolism is associated with reduced substrate delivery to the mitochondria (through the action of pyruvate dehydrogenase kinase 1 [PDK1]) and reduced mitochondrial mass (as a result of autophagy triggered by BNIP3). As described by Sonveaux et al. in their study in this issue of the JCI (11), aerobic tumor cells express proteins that allow them to take up lactate (e.g., MCT1) and use it (e.g., LDHB), in the presence of O2, as their principal substrate for mitochondrial oxidative phosphorylation. Note that hypoxic cells generate 2 mol of ATP and 2 mol of lactate per mol of glucose consumed, whereas aerobic cells generate 36 mol of ATP per 2 mol of lactate consumed. pO2, partial pressure of oxygen.