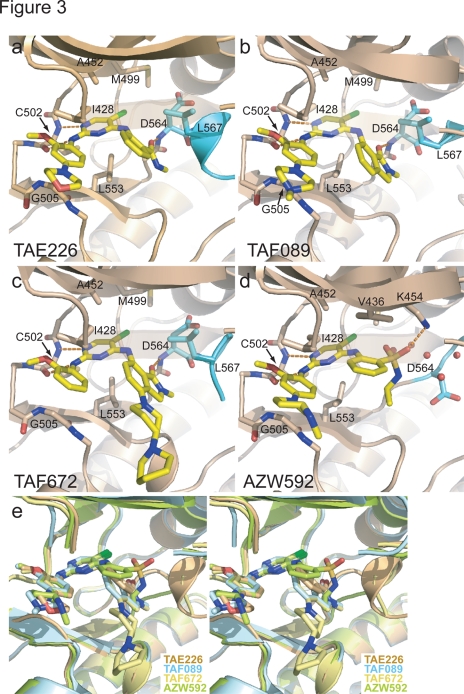
Figure 3. Drug binding mode of the FAK inhibitors.
(a–d). The inhibitors TAE226 (a), TAF089 (b), TAF672 (c) and AZW592 (d) are shown bound to the active site of the FAK kinase (beige ribbon with activation loop in cyan). Key side chains and the inhibitors (yellow) are shown in stick representation. For clarity residues 429–431 in the P-loop are rendered transparent. (e) Stereoview of the superposition of the four structures displayed in (a–d). For clarity residues 429–431 in the P-loop are removed. The substitutions at the 4-aniline ring of TAE226, TAF089 and TAF672 induce a helical conformation of the DFG motif, whereas AZW592 bound FAK exhibits an extended activation loop in this region.