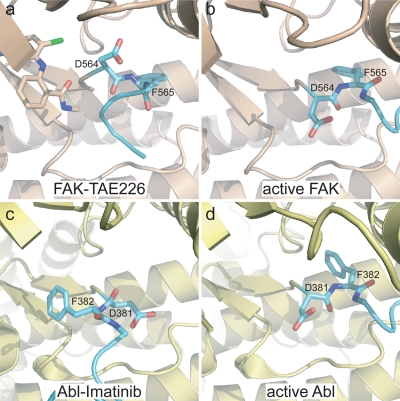
Figure 4. Partial DFG flip in TAE226 bound FAK.
(a–b). The phi backbone torsion angle of D564 in TAE226 bound FAK (a) is rotated by approximately 110° compared to FAK in the active conformation (b) (pdb accession 2j0l). This results in the side chain of D564 pointing upwards towards the N-lobe. FAK is colored beige with its activation loop in cyan. TAE226 (a) and the DFG motif are shown in stick representation. For clarity, residues 429–434 in the P-loop and AMP-PNP in active FAK are removed. (c–d) In Abl, D381 of the DFG motif is flipped by approximately 180° in the imatinib bound form (c) (pdb accession 1opj) compared to the active conformation (d) (pdb accession 2g2i). Abl is colored pale yellow with its activation loop in cyan. The ligands imatinib (c) and ADP (d) as well as residues 251–255 in the P-loop are removed because they obstruct the view of the DFG motif.