Abstract
Objectives
In a prospective, randomized trial, daptomycin was non-inferior to standard therapy for Staphylococcus aureus bacteraemia and right-sided endocarditis. Since rates of infection due to methicillin-resistant S. aureus (MRSA) infection are increasing and treatment outcomes for bacteraemia caused by MRSA are generally worse than those observed with methicillin-susceptible S. aureus bacteraemia, clinical characteristics and treatment results in the trial’s pre-specified subset of patients with MRSA were analysed.
Methods
Clinical characteristics and outcomes of patients receiving daptomycin were compared with those receiving vancomycin plus low-dose gentamicin. Success was defined as clinical improvement with clearance of bacteraemia among patients who completed adequate therapy, received no potentially effective non-study antibiotics and had negative blood cultures 6 weeks after end of therapy.
Results
Twenty of the 45 (44.4%) daptomycin patients and 14 of the 43 (32.6%) vancomycin/gentamicin patients were successfully treated (difference 11.9%; confidence interval −8.3 to 32.1). Success rates for daptomycin versus vancomycin/gentamicin were 45% versus 27% in complicated bacteraemia, 60% versus 45% in uncomplicated bacteraemia and 50% versus 50% in right-sided MRSA endocarditis. Cure rates in patients with septic emboli and in patients who received pre-enrolment vancomycin were similar between treatment groups. However, in both treatment groups, success rates were lower in the elderly (≥75 years). Persisting or relapsing bacteraemia occurred in 27% of daptomycin and 21% of vancomycin/gentamicin patients; among these patients, MICs of ≥2 mg/L occurred in five daptomycin and four vancomycin/gentamicin patients. The clinical course of several patients may have been influenced by lack of surgical intervention.
Conclusions
Daptomycin was an effective alternative to vancomycin/gentamicin for MRSA bacteraemia or right-sided endocarditis.
Keywords: MRSA, endovascular infections, bloodstream infections, combination therapy, clinical trial
Introduction
Staphylococcus aureus is the most common cause of infective endocarditis in many regions of the world1 and in the USA is second only to coagulase-negative staphylococci in causing bacteraemia.2 Since it was first described in 1961, methicillin-resistant S. aureus (MRSA) has become a common healthcare- associated pathogen, causing one-third of the cases of S. aureus endocarditis in the USA.1 In recent years, a particularly virulent clone of community-associated MRSA has been associated with significant disease in non-healthcare-associated settings,3,4 and it has found its way into hospitals as a cause of healthcare- associated bacteraemias.5–7
Numerous studies have compared the characteristics of methicillin-susceptible S. aureus (MSSA) and MRSA bacteraemia and endocarditis. Fowler et al.8 found that patients with MRSA endocarditis were more likely to have underlying diabetes mellitus, an immunocompromised state, recent invasive procedure, presumed intravenous (iv) source, healthcare-associated disease and persistent bacteraemia. For reasons that may relate to the bacterial strain, the patient population or the antimicrobials used for therapy, the duration of bacteraemia in MRSA bloodstream infections and endocarditis is, on average, longer than that associated with MSSA.1,8,9
While the morbidity and mortality associated with both MSSA and MRSA bacteraemia and endocarditis are notable, the rates of complicated disease and death among individuals infected with MRSA are higher, even after controlling for the increased numbers of co-morbidities in the MRSA group.10,11 This phenomenon was also observed among patients participating in the recent randomized trial of daptomycin versus standard therapy for these conditions.8 Whether lower treatment success rates are due to the microorganism or the antimicrobials used to treat the infection remains unknown. The suboptimal treatment results observed when vancomycin rather than semi-synthetic penicillin is used to treat MSSA bacteraemia and endocarditis12–15 leads to speculation with regard to the potency of vancomycin and its potential impact on the outcomes of treatment of serious MRSA infections. The availability of other antimicrobial agents with activity against MRSA provides an opportunity to examine alternative regimens. The aim of the study was to compare the clinical characteristics and outcomes of patients with MRSA bacteraemia or endocarditis treated with daptomycin with those of patients treated with vancomycin and short-course gentamicin.
Methods
Design overview
Patients participated in this open-label, multicentre, international, randomized trial between August 2002 and March 2005. The trial is registered on ClinicalTrials.gov (NCT00093067). The parent study was a non-inferiority trial to assess the efficacy and safety of daptomycin compared with standard therapy (either an antistaphylococcal penicillin or vancomycin, with initial short-course gentamicin) for MSSA and MRSA bacteraemia and endocarditis.8 Patients were randomized by a computerized central randomization schedule to either daptomycin or standard therapy in a 1:1 ratio. The primary outcome was the clinical success rate at the test of cure visit 42 days after completion of therapy. The null hypothesis was that the treatments differed by at least 20%. The central database of this trial was used to perform a detailed analysis of the pre-specified subset of patients with MRSA bacteraemia or endocarditis.
Setting and participants
The protocol, approved by the Institutional Review Board at each site, was consistent with the principles of the Declaration of Helsinki. To improve comparability among patients and to accommodate the guidelines for a registrational trial, some patients were excluded from randomization into the trial. Eligible patients were ≥18 years of age with one or more blood cultures growing S. aureus within 2 calendar days prior to randomization. Patients whose creatinine clearance was <30 mL/min and those with known osteomyelitis, polymicrobial bacteraemia or pneumonia were ineligible for participation. Prior receipt of an antibiotic active against S. aureus was not an exclusion criterion. Patients with prosthetic valves were ineligible to participate, as were those who had intravascular foreign material at the time a positive blood culture was drawn (unless the investigator intended to have the material removed within 4 days after the first dose of study medication). All patients or their authorized representatives provided written informed consent.
Interventions: MRSA subset
Participants with MRSA bacteraemia or endocarditis were to receive either daptomycin 6 mg/kg iv every 24 h or standard therapy (vancomycin 1 g iv every 12 h with gentamicin 1 mg/kg iv every 8 h for the first 4 days of treatment).16 Vancomycin and gentamicin doses were adjusted by investigators. Blood cultures were performed daily until they were negative, at the end of therapy and 42 days after completion of therapy (test of cure). Haematological parameters, coagulation tests, creatine phosphokinase levels and chemistry profiles were monitored during therapy and at the test of cure visit.
Definitions and outcomes
The investigators established an initial diagnosis based on the modified Duke criteria17 and determined the duration of therapy according to their working diagnosis. Complicated bacteraemia was present when MRSA was isolated from the blood on at least 2 days during the first 5 days of therapy, metastatic infection was found or the infection involved prostheses that were not removed within 4 days. These patients, who did not have endocarditis as defined by the Duke criteria, were to receive a minimum of 28 days of therapy. Uncomplicated bacteraemia was usually treated for 10–14 days. If patients had definite or possible endocarditis17 in the absence of predisposing abnormalities or active lesions of the aortic or mitral valves, the diagnosis of right-sided endocarditis was established. Left-sided endocarditis was defined as described in the modified Duke criteria.17 The planned treatment course for patients with endocarditis was a minimum of 28 days.
The final diagnoses and outcomes were determined by an independent adjudication committee blinded to treatment.8 Conflicts of opinion between members of the committee were settled by discussion leading to consensus. Persisting or relapsing bacteraemia was investigator-defined, meaning that the investigator decided when to discontinue study medication in the case of ongoing or recurrent MRSA bacteraemia. The independent adjudication committee reviewed the role of persisting or relapsing MRSA bacteraemia in its analysis of outcomes.
The treatment outcome was categorized as success if an adequately treated patient was clinically cured or improved and blood cultures were negative 42 days after completion of therapy. The outcome was categorized as failure if a patient experienced persisting or relapsing MRSA infection, clinical failure or death for any reason. Failure was also defined by premature discontinuation of therapy because of a treatment-limiting adverse event, receipt of potentially effective non-study antibiotics or lack of blood culture at the 42 day post-therapy evaluation.
Statistical analysis
Differences between the patients in the treatment groups were analysed using Fisher’s exact test for categorical data (occurrence of adverse events and laboratory abnormalities) and analysis of covariance for continuous data (change from baseline serum creatinine). The 95% confidence interval (CI) for the difference in success rates (daptomycin minus vancomycin/gentamicin) was calculated based on the normal approximation to the binomial distribution. Survival curves were estimated using the Kaplan–Meier methodology, and the treatment groups were compared using the Wilcoxon and log-rank tests. All analyses were performed using SAS® software, Version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Among the 89 patients with MRSA bacteraemia or endocarditis, 45 received daptomycin, 43 were given vancomycin/gentamicin and 1 was inadvertently treated with a semi-synthetic penicillin. The latter patient was excluded from further data analyses.
Table 1 summarizes the baseline demographic information for the MRSA patients. The treatment groups were well balanced with regard to clinical characteristics. Twice as many patients who received daptomycin were 75 years of age and older (22% versus 9%), but this difference was not statistically significant.
Table 1.
Demographics, baseline characteristics and final diagnosis of MRSA patients
| Daptomycin (n = 45) | Vancomycin/gentamicin (n = 43) | |
|---|---|---|
| Age (years), median (range) | 57 (22–86) | 54 (25–91) |
| Gender (male), n (%) | 23 (51) | 25 (58) |
| Race, n (%) | ||
| black | 10 (22) | 9 (21) |
| Caucasian | 31 (69) | 29 (67) |
| Hispanic | 3 (7) | 2 (5) |
| other | 1 (2) | 3 (7) |
| Weight (kg), median (range) | 70.5 (52.0–119.4) | 71.5 (49.9–119.9) |
| BMI (kg/m2), median (range) | 26.5 (18.6–49.6) | 25.4 (18.9–44.0) |
| Creatinine clearance (mL/min), median (range) | 77.6 (29.4–200.8) | 76.3 (17.9–171.0) |
| Underlying conditions, n (%) | ||
| injection drug use | 12 (27) | 11 (26) |
| diabetes mellitus | 17 (38) | 18 (42) |
| septic pulmonary emboli | 4 (9) | 5 (12) |
| HIV infection | 2 (4) | 0 |
| history of endocarditis | 3 (7) | 3 (7) |
| underlying valvular heart disease | 9 (20) | 6 (14) |
| presence of foreign material | ||
| intravascular | 5 (11) | 9 (21) |
| extravascular | 14 (31) | 14 (33) |
| SIRS | 35 (78) | 33 (77) |
| Final diagnosis, n (%)a | ||
| uncomplicated bacteraemia | 10 (22) | 11 (26) |
| complicated bacteraemia | 22 (49) | 22 (51) |
| right-sided endocarditis | 8 (18) | 6 (14) |
| left-sided endocarditis | 5 (11) | 4 (9) |
BMI, body mass index; HIV, human immunodeficiency virus; SIRS, systemic inflammatory response syndrome.
aAs determined by the adjudication committee.
Underlying medical conditions were also similar between the treatment groups (Table 1). More than one-third of the patients had diabetes mellitus, and injection drug use was present in one-quarter of the patients. The systemic inflammatory response syndrome was observed among three-quarters of the patients in both groups. Four patients who received daptomycin and five who received vancomycin/gentamicin had septic pulmonary emboli at baseline (9% and 11%, respectively).
The final diagnoses, as established by the adjudication committee, are summarized in Table 1. Half of the patients in each group had complicated MRSA bacteraemia without documented endocarditis, and approximately one-quarter were diagnosed with uncomplicated bacteraemia. In the daptomycin arm, eight patients had right-sided and five had left-sided endocarditis, compared with six with right-sided and four with left-sided endocarditis in the vancomycin/gentamicin arm.
The mean and median trough vancomycin levels were 14.9 and 14.7 mg/L, respectively. Thirty-nine of the 43 patients (91%) in the vancomycin/gentamicin arm received a mean of 4.3 days of initial gentamicin therapy as part of the study protocol.
Six weeks following the conclusion of therapy, the overall success rate was 44.4% for patients who received daptomycin and 32.6% for patients in the vancomycin/gentamicin arm (difference in rates 11.9%; CI −8.3 to 32.1) (Table 2). The success rates in the treatment of complicated bacteraemia were 45% in the daptomycin group and 27% in the vancomycin/gentamicin group. For patients with uncomplicated bacteraemia, success rates were 60% for daptomycin and 45% for vancomycin/gentamicin. Four of eight patients receiving daptomycin and three of six receiving vancomycin/gentamicin were successfully treated for right-sided endocarditis. Of those with septic pulmonary emboli, three of the four daptomycin-treated patients and three of the five vancomycin/gentamicin-treated patients were considered cures. None of the nine patients with left-sided endocarditis was successfully treated.
Table 2.
Treatment success rates for MRSA
| Bacteraemiaa |
Endocarditisa |
Totala,b | |||
|---|---|---|---|---|---|
| uncomplicated | complicated | right-sided | left-sided | ||
| Daptomycin | 6/10 (60%) | 10/22 (45%) | 4/8 (50%) | 0/5 (0%) | 20/45 (44.4%) |
| Vancomycin/gentamicin | 5/11 (45%) | 6/22 (27%) | 3/6 (50%) | 0/4 (0%) | 14/43 (32.6%) |
aSuccess/number treated.
bDifference in success rates=11.9% (CI −8.3 to 32.1).
Success rates were somewhat lower among older patients. Among patients younger than 75 years of age, 18 of 35 (51.4%) in the daptomycin arm and 14 of 39 (35.8%) in the vancomycin/gentamicin group were judged to have had a successful outcome, but among patients 75 years of age or older, 2 of the 10 receiving daptomycin and none of the 4 in the vancomycin/gentamicin arm were successfully treated. The two patients in the older age group who were treated successfully with daptomycin had complicated bacteraemia; the remainder who received daptomycin were diagnosed with uncomplicated bacteraemia (one patient), complicated bacteraemia (four patients) and left-sided endocarditis (three patients). The diagnoses for patients 75 years of age and older who received vancomycin/gentamicin were complicated bacteraemia (three patients) and right-sided endocarditis (one patient).
Fourteen patients had intravascular material in place at the time of randomization. Ten of the 14 had pacemakers, defibrillators or tunnelled catheters, and 4 of these 10 devices were removed. Overall, two of five daptomycin-treated patients and three of the nine who received vancomycin/gentamicin were treated successfully.
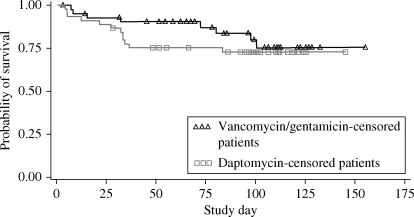
The overall death rate among patients in the daptomycin group was 12/45 (27%), compared with 8/43 (19%) for the vancomycin/gentamicin group (P = 0.45). A Kaplan–Meier plot (Figure 1) demonstrates no significant differences in survival through day 150 (Wilcoxon P = 0.25, log-rank P = 0.42). Among patients with persistent or relapsing MRSA bacteraemia, 7 of the 12 receiving daptomycin (58%) and 6 of 9 (67%) in the vancomycin/gentamicin group died (Table 3).
Figure 1.
The Kaplan–Meier plot of overall survival. Wilcoxon P= 0.25, log-rank P = 0.42.
Table 3.
Patients with persisting/relapsing MRSA infection
| Diagnosis | Days of therapy | Complications/interventions | Ultimate outcome |
|---|---|---|---|
| Daptomycin | |||
| cBAC | 3 | pacemaker, prosthetic hip; pacemaker not removed | care withdrawn, died |
| 3 | chronic osteomyelitis of humerus, prosthesis in situ; no surgery | died | |
| 4 | cardiogenic shock, aortic balloon pump; central venous catheter infection, possible septic thrombophlebitis; catheter not removed | died | |
| 9 | undiagnosed mycotic aneurysm of thoracic aorta, progression of myeloma; no surgery | died | |
| 13 | psoas abscess, vertebral osteomyelitis; abscess drained 3 days after course of study medication completed | died | |
| 14 | infected subcutaneous intravenous port, possible septic thrombophlebitis; port removed day 2, pocket inadequately debrided | cured with additional antibiotics | |
| 23 | septic arthritis; knee aspirated day 2, synovectomy day 5 and again 20 days after end of study medication when relapse diagnosed | cured with additional antibiotics | |
| 35 | pancreas transplant, unrecognized retroperitoneal abscess; abscess diagnosed day 31, drainage attempted day 32 | cured with additional antibiotics | |
| LIE | 6 | prosthetic aortic IE, sternal osteomyelitis; re-do aortic valve replacement, sternal debridement 2 days after end of study medication | cured with additional antibiotics |
| 7 | mitral/aortic IE, pulmonary oedema; no surgery | made DNR, died | |
| 8 | mitral IE, stroke; no surgery | died | |
| 14 | infected lumbar spine prosthesis, aortic valve endocarditis; L2-3 debridement, hardware removed 11 days after end of study medication | cured with additional antibiotics | |
| Vancomycin/gentamicin | |||
| cBAC | 3 | decubitus ulcers, renal failure, sepsis; no debridement | died |
| 5 | septic thrombophlebitis, osteomyelitis; no surgery | cured with additional antibiotics | |
| 13 | cutaneous T cell lymphoma, infected ulcers; no debridement | died | |
| 15 | high grade SAB, psoas and scrotal abscesses, MRSA pneumonia; inadequate drainage of abscesses | cured with additional antibiotics | |
| 26 | enterocutaneous fistulae, inadequately drained subphrenic abscesses; septic thrombophlebitis | cured with additional antibiotics | |
| RIE | 3 | pacemaker IE, right atrial vegetation, sepsis, acute renal failure; pacemaker not removed | died |
| 7 | pacemaker endocarditis; pacemaker not removed | died | |
| LIE | 7 | aortic IE, septic emboli (retina, spleen), septic shock; no surgery | died |
| 27 | mitral IE, intramural abscess, stroke; no surgery | care withdrawn, died | |
cBAC, complicated bacteraemia; DNR, do not resuscitate; IE, infective endocarditis; LIE, left-sided endocarditis; RIE, right-sided endocarditis; SAB, S. aureus bacteraemia.
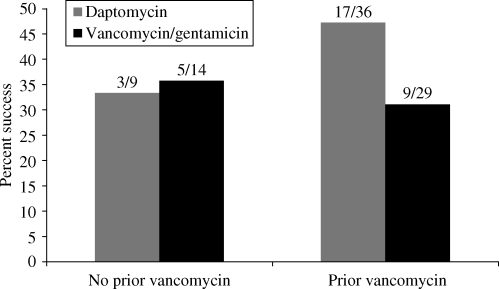
Because of recent in vitro studies suggesting that exposure of S. aureus to vancomycin may reduce susceptibility to daptomycin18 and reports of trends toward rising vancomycin MICs,19 we reviewed pre-treatment and baseline MICs. Daptomycin isolates with MICs ≤1 mg/L were considered susceptible; the susceptibility cut-off for vancomycin MICs was ≤2 mg/L.20 Thirty-six of the 45 patients (80%) who received daptomycin and 29 of the 43 (67%) patients taking vancomycin/gentamicin had received vancomycin for 1–22 days (mean and median, 3 days) within 30 days prior to study entry. Five patients in the daptomycin arm and four in the vancomycin arm received ≥5 days of vancomycin prior to study entry. In the daptomycin group, the baseline MIC for daptomycin was 0.25 mg/L for 35/45 (78%) patients and 0.5 mg/L for the remaining 10 patients. The baseline vancomycin MICs were 0.5 mg/L for 22 of 43 patients (51%) and 1 mg/L for 21 of 43 patients (49%). There was no evidence of any impact of prior vancomycin treatment on baseline MICs. Likewise, there was no evidence of reduced success rates with vancomycin pre-treatment (Figure 2).
Figure 2.
Effect of prior vancomycin treatment.
Twelve of the 45 (27%) daptomycin patients and 9 of the 43 (21%) vancomycin/gentamicin patients experienced persisting or relapsing MRSA bacteraemia (Table 3). Several of these patients were immunocompromised; many had deep-seated foci of infection that might have benefited from surgical intervention but they were deemed poor surgical candidates due to their overall condition. There was no correlation between receipt of vancomycin prior to study entry and subsequent development of persisting or relapsing MRSA bacteraemia. Eight of the 12 patients (67%) in the daptomycin group who failed therapy related to persistence or relapse had prior vancomycin therapy, compared with a rate of prior vancomycin exposure of 28/33 (85%) among daptomycin patients who did not (P = 0.22). Similarly, for those randomized to receive vancomycin/gentamicin, 4/9 (44%) of the patients who did and 25/34 (74%) of those who did not experience persisting or relapsing MRSA bacteraemia had previous vancomycin therapy (P = 0.12). Among patients who failed therapy because of persistence or relapse of bacteraemia, isolates from 5 of the 12 in the daptomycin arm had an MIC increase to 2 mg/L in cultures drawn a median of 7 (mean 17) days after study initiation. Likewise, isolates from four of the nine patients in the vancomycin/gentamicin arm had an MIC increase to 2 mg/L at the local or central laboratory, found a median of 16 (mean 27) days after randomization. The difference in time to documentation of isolates with increased MICs was influenced by variations in frequency of sampling and the fact that two isolates from the daptomycin group were from patients with left-sided endocarditis who were not surgical candidates and experienced early failure of therapy.
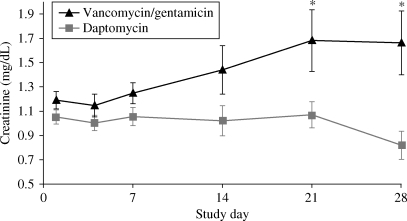
Among investigator-reported adverse events, the rates of nausea and anaemia were significantly higher (P = 0.03 and 0.05, respectively; Fisher’s exact test) in vancomycin/gentamicin-treated patients. With regard to laboratory abnormalities, serum creatinine levels trended higher among those in the vancomycin/gentamicin arm. At days 21 and 28, there was a statistically significant difference between mean serum creatinine levels of patients receiving vancomycin/gentamicin compared with those receiving daptomycin (Figure 3). The higher incidence of creatine phosphokinase elevations >500 U/L among daptomycin-treated patients did not translate into more reports of musculoskeletal adverse events (Table 4).
Figure 3.
Mean serum creatinine. *P ≤ 0.05 compared with daptomycin for change from baseline. Bars represent the standard error of the means.
Table 4.
Muscle effects
| Daptomycin (n = 45) n (%) | Vancomycin/gentamicin (n = 43) n (%) | P valuea | |
|---|---|---|---|
| Reported clinical adverse events | |||
| CPK increased | 2 (4) | 0 | 0.49 |
| musculoskeletal events | 11 (24) | 20 (47) | 0.04 |
| discontinued therapy due to CPK | 1 (2) | 0 | >0.99 |
| Lab abnormalities | |||
| CPK shift from normal to >500 IU/L | 4/38 (11) | 0/36 | 0.12 |
CPK, creatine phosphokinase.
aFisher’s exact test.
A total of 3 of the 45 patients in the daptomycin arm (7%) failed therapy because of treatment-limiting adverse events (one patient each with vomiting, diabetic gastroparesis and renal failure), compared with 7 of the 43 patients in the vancomycin/gentamicin arm (16%), in which renal dysfunction was the most common reason for failure due to treatment-limiting adverse events.
Discussion
The treatment of bacteraemia and endocarditis caused by MRSA continues to be challenging. Despite advances in diagnostic techniques and supportive care, patients with bacteraemia may develop suppurative complications and metastatic infections. In particular, the development of infective endocarditis is feared. Patients may become unstable so quickly that they become ineligible for otherwise life-saving surgical therapy. For many years, there have been no significant advances related to antimicrobial therapy for MRSA bacteraemia and endocarditis.
The current study, the only prospective and randomized trial comparing a newly available antibiotic with standard therapy for MRSA bacteraemia and endocarditis, provides a unique opportunity to address questions about the role of antimicrobials in treating these life-threatening infections. In this analysis of patients with MRSA bacteraemia and endocarditis, daptomycin performed as well as vancomycin/gentamicin, with fewer adverse events. Even among older patients, a group at higher risk of morbidity and mortality, daptomycin performed at least as well as standard therapy. Success rates among patients with septic pulmonary emboli were similar in both treatment arms. This result is consistent with experimental models demonstrating the efficacy of daptomycin for haematogenous S. aureus infections of the lung.21
Because stringent criteria were used to define outcomes, the overall success rates in both treatment arms were low. Some of the reasons for failure, such as lack of adherence to protocol or discontinuation of therapy because of adverse events, were related to the study format. Several patients who were considered failures secondary to persisting or relapsing bacteraemia were found to have deep-seated foci of infection, including osteomyelitis, infected devices and abscesses that were not apparent at the time of study entry.
Prior vancomycin therapy did not appear to influence the treatment response, a potentially significant finding in view of in vitro studies suggesting potential increases in daptomycin and vancomycin MICs among S. aureus strains exposed to vancomycin.18,22,23 The lack of success of therapy for patients with left-sided endocarditis in both treatment arms is of concern but difficult to interpret in light of the small number of patients with endocarditis in this study. Several of these patients, as well as others with right-sided endocarditis or complicated bacteraemia, failed therapy because treating physicians determined that they were not candidates for surgical intervention. The fact that most of the patients in the vancomycin arm received concomitant short-course gentamicin therapy may have influenced the rate of nephrotoxicity in this group; an analysis is under review. Recent guidelines state that the addition of gentamicin for therapy of S. aureus native valve endocarditis is optional.16
The reasons that MRSA bacteraemia and endocarditis are so difficult to treat are multifaceted. They are related to the patient populations who develop infections, characteristics of MRSA and the traits of the antibiotics used to treat MRSA.
Host factors certainly impact outcomes10 and there has been a change in the types of patients developing S. aureus bacteraemia and endocarditis: chronic illness and exposure to healthcare interventions are now more common risk factors than injection drug use.1 Co-morbid conditions such as age, diabetes mellitus, chronic indwelling central catheters and paravalvular complications have been shown to predict mortality, particularly among patients with left-sided endocarditis.24–26
The issue of reduced susceptibility of MRSA to specific antibiotics illustrates the role of organism-specific traits in response to therapy. There are difficulties in defining antibiotic susceptibility and resistance,27 but there has been a general rise in vancomycin MICs among S. aureus,19 with an associated increased failure rate of vancomycin in treating S. aureus bacteraemia when the vancomycin MIC is in the upper end of the ‘susceptible’ range.28–33 Most clinical microbiology laboratories do not screen MRSA isolates for vancomycin heteroresistance, which is not detected with automated susceptibility testing.34 Importantly, in the current study, the baseline MIC testing of the isolates showed that they were uniformly susceptible to daptomycin (0.25–0.5 mg/L) and vancomycin (0.5–1 mg/L). The MICs for strains that are initially susceptible may rise during therapy; for daptomycin, increases in MICs appear to involve genetic changes at several sites.35
Finally, factors associated with the antimicrobials used to treat MRSA bacteraemia and endocarditis have been examined in an effort to explain the excessive mortality observed in these conditions.9,12,36 Since the bactericidal activity of vancomycin is time-dependent, some have suggested that higher trough levels (i.e. in the range of 15–20 mg/L or higher) might be useful,37 but recent reports demonstrated increased toxicity without improvement in outcome when high-dose vancomycin was used to treat MRSA infections.29,38 Vancomycin tolerance has been associated with poor outcomes.14,39,40 Among patients with left-sided native valve endocarditis due to S. aureus, timely surgical intervention may be at least as important as antimicrobial therapy.24,26
There are several potential limitations to this report, including the fact that it represents a pre-specified subset analysis rather than a prospective, blinded study. Patients with renal failure and those with prosthetic devices or long-term indwelling venous catheters that could not be removed were to be excluded. During the course of therapy, foci of infection such as abscess, osteomyelitis and indwelling devices became apparent in several cases; the type and timing of surgical intervention that might have impacted outcomes was not standardized. Persisting and relapsing bacteraemia was investigator-defined, and we were unable to make a statement with regard to the mean duration of bacteraemia because of the limitations in collecting daily blood cultures. The number of patients with left-sided endocarditis due to MRSA was small, and there were no treatment successes in this group. In this study, the daptomycin MICs for baseline and subsequent isolates were confirmed at a central laboratory, but the vancomycin MICs for subsequent isolates were usually determined by local laboratories.
Conclusions
Cases of bacteraemia and endocarditis due to MRSA continue to be associated with high rates of morbidity and mortality. The reasons are multifactorial, including underlying patient conditions, characteristics of S. aureus itself and issues related to vancomycin, the historical gold standard for treatment of serious MRSA infections. Daptomycin appears to be a therapeutic option for patients with MRSA bacteraemia and right-sided endocarditis.
Funding
This study was sponsored and funded by Cubist Pharmaceuticals Inc., Lexington, MA, USA.
Transparency declarations
S. J. R. serves on scientific advisory boards for Cubist and Pfizer, as a speaker for Cubist and Wyeth and has received honoraria and grants from Cubist, Pfizer and Wyeth. H. B. serves as an advisor/consultant to Astellas, Astra-Zeneca, Basilea, Cubist, Johnson & Johnson, Merck, Novartis, Pfizer, Targanta and Theravance, has been a speaker for Cubist, Pfizer and Schering-Plough and owns or has owned shares of Pfizer and Cubist. D. L. serves as a consultant and speaker for Cubist, has received honoraria and grants from Cubist and serves as an advisor/consultant to Theravance and Astellas. M. C. is an employed consultant for Cubist and owns Cubist stock; she is also a consultant for Aeris Therapeutics, Dyax Corporation, Shire Human Genetic Therapies and Cowen and Company. B. I. E. is an employee of Cubist and owns Cubist stock. G. A. V. was formerly employed by Cubist and owns Cubist stock. G. R. C. serves as a consultant to Cubist, Theravance, Merck, Pfizer and Innocol and has received honoraria and grants from Cubist.
The statistical analyses were performed by M. C., a consultant for Cubist, under the direction of the authors. The funding source reviewed the data in the manuscript for accuracy. The authors had full access to all the data in the study, interpreted the data, created the manuscript and had final responsibility for the decision to submit for publication.
Acknowledgements
This paper is dedicated to the memories of Frank P. Tally, MD, and Elias Abrutyn, MD, clinician-scholars who made extraordinary contributions to our understanding of the treatment of S. aureus infections.
In addition to the authors, the following investigators participated in the trial: R. Akins, H. Albrecht, M. Barron, J. M. Bernstein, M. Bessesen, H. R. Brodt, C. H. Cabell, P. Carson, H. F. Chambers, P. Cook, S. E. Cosgrove, I. DeMeyer, G. Fätkenheuer, D. Fierer, S. G. Filler, M. Foltzer, G. N. Forrest, V. G. Fowler, Jr, M. Gareca, D. Goodenberger, K. High, B. Hirsch, B. Hoen, C. Hsiao, A. W. Karchmer, H. Lampris, T. P. Le, M. Levinson, A. S. Link, J. Parsonnet, W. Peetermans, D. Pitrak, C. S. Price, J. P. Quinn, J. Ramirez, P. A. Rice, M. E. Rupp, B. Salzberger, J. Segreti, J. Stone, B. Suh, J. Tan (deceased), F. P. Tally (deceased), Z. Temesgen, A. Tice and M. Zervos.
References
- 1.Fowler VG, Jr, Miro JM, Hoen B, et al. ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Fridkin SK, Hageman JC, Morrison M, et al. Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 4.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 5.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–56. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 6.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 7.Boucher HG, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S344–9. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VG, Jr, Boucher HW, Corey GR, et al. S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 9.Levine D, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–80. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–9. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 11.Shurland S, Zhan M, Bradham DD, et al. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:273–9. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 12.Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine. 2003;82:333–9. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 13.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–6. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 14.Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;6:1227–31. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S-H, Kim K-H, Kim H-B, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–7. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 17.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 18.Sakoulas G, Alder J, Thauvin-Eliopoulos C, et al. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother. 2006;50:1581–5. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Hindler JF, Ward KW, et al. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–6. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Sixteenth Informational Supplement M100-S16. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 21.Silverman JA, Mortin LI, Van Praagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–52. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 22.Sakoulas G, Rose W, Rybak M, et al. Evaluation of endocarditis caused by methicillin-susceptible Staphylococcus aureus developing nonsusceptibility to daptomycin. J Clin Microbiol. 2008;46:220–4. doi: 10.1128/JCM.00660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moise PA, Smyth DA, El-Fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61:85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy O, Sexton DJ, Wang A, et al. Early surgery in patients with infective endocarditis: a propensity score analysis. Clin Infect Dis. 2007;44:364–72. doi: 10.1086/510583. [DOI] [PubMed] [Google Scholar]
- 25.Hasbun R, Vikram HR, Barakat LA, et al. Complicated left-sided native valve endocarditis in adults: risk classification for mortality. JAMA. 2003;289:1933–40. doi: 10.1001/jama.289.15.1933. [DOI] [PubMed] [Google Scholar]
- 26.Vikram HR, Buenconsejo J, Hasbun R, et al. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA. 2003;290:3207–14. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother. 2003;47:3040–5. doi: 10.1128/AAC.47.10.3040-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakoulas G, Moise-Broder PA, Schentag J, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidayat LK, Hsu DI, Quist Q, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166:2138–44. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 30.Macclayton DO, Suda KJ, Coval KA, et al. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 µg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin Ther. 2006;28:1208–16. doi: 10.1016/j.clinthera.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Jones RN. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin Infect Dis. 2006;42:S13–24. doi: 10.1086/491710. [DOI] [PubMed] [Google Scholar]
- 32.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 33.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with MRSA bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 35.Friedman L, Alder J, Silverman J. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:2137–45. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howden BP, Johnson PD, Ward PB, et al. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:3039–47. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybak M. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42:S35–9. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 38.Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–6. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakoulas G, Gold HS, Cohen R, et al. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother. 2006;57:699–704. doi: 10.1093/jac/dkl030. [DOI] [PubMed] [Google Scholar]
- 40.Safdar A, Rolston KVI. Vancomycin tolerance, a potential mechanism for refractory Gram-positive bacteremia: observational study in patients with cancer. Cancer. 2006;106:1815–20. doi: 10.1002/cncr.21801. [DOI] [PubMed] [Google Scholar]