Abstract
Bone marrow stem cells (BMSCs) are pluripotent cells that have been used to facilitate bone repair because of their capability of differentiating into osteoblasts. However, it is well known that the number of BMSCs with osteogenic potential decreases in patients with old age, osteoporosis, and metabolic diseases. In such conditions, xenogenic BMSCs may provide an alternative to autologous BMSCs. In the current study, we investigated the potential of transplanted xenogenic BMSCs to survive and generate new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits. The BMSCs were obtained from bilateral femurs of four male rats, cultured and expanded in medium with osteoinduction supplement. The BMSCs (1,000,000 cells) of male rats loaded onto 5 cc compression resistant matrix (CRM; Medtronic Sofamor Danek, USA) were implanted bilaterally onto the L4-5 intertransverse processes of 16 female rabbits (xenogenic BMSCs + CRM group). The 16 female rabbits that received 5 cc CRM alone were used as controls (CRM alone group). To exclude the possibility of migration of BMSCs from the transverse processes of the recipient rabbits, we did not decorticate the transverse processes. No rabbits received any immunosuppressive medications during the experiment. Four rabbits each in both of the experimental and control groups were killed at 1, 2, 4, and 6 months postimplantation, and the lumbar spine underwent radiological and histological analyses for evaluation of new bone formation. The polymerase chain reaction (PCR) for Sry gene (Y-chromosome-specific marker) was used to evaluate the survival of transplanted xenogenic BMSCs. The expression of Sry gene was clearly identified in the lumbar spines of all the 16 rabbits in the xenogenic BMSCs + CRM group at 1–6 months postimplantation. Serial plain radiographs showed gradual resorption of CRM; however, it was difficult to clearly identify the presence of new bone formation due to the radiopacity of the remaining CRM. Histologically, mature lamellar and woven bone with osteoblasts and osteocytes were identified in all eight rabbits in the xenogenic BMSCs + CRM group at 4 and 6 months postimplantation, but in none of the eight rabbits at 1 and 2 months postimplantation. None of CRM alone group showed new bone formation at 1–6 months postimplantation. Mild-to-moderate infiltration of inflammatory cells was identified around the CRM carriers in both the groups. No post-operative wound infection was found in either group. Our results indicate that xenogenic BMSCs loaded onto CRM survive and generate new bone formation when placed into the posterolateral lumbar spine of rabbits without immunosuppression. To determine if a solid fusion can be achieved with such techniques, further studies are needed to investigate the appropriate dose of xenogenic BMSCs, amounts of CRM, and the requisite incubation time.
Keywords: Xenogenic bone marrow stem cells, Survival, Bone formation, Immune suppression, Posterolateral lumbar spine
Introduction
The use of autogenous bone graft has been considered as the “gold standard” for obtaining spinal fusion because of its osteoinductive, osteogenic, and osteoconductive properties. However, harvesting autogenous bone graft is inevitably associated with donor site morbidities and increased surgical time [27]. Therefore, extensive basic and clinical researches have been directed at developing alternatives to autogenous bone graft [11, 15, 17, 18, 21, 26].
Bone marrow stem cells (BMSCs) are pluripotent cells that have been used to facilitate bone repair because of their capability of differentiating into osteoblasts. Because of their proliferative capacity, BMSCs can be isolated from a small amount of bone marrow aspirate and expanded in culture into billions of cells. Moreover, BMSCs can be subcultured for as many as 15 passages and cryopreserved without losing their differentiation potential [4–6, 8, 13, 16, 23]. However, it is well known that the number of BMSCs with osteogenic potential decreases in patients with old age, osteoporosis, and metabolic diseases [7, 10]. In such conditions, the clinical use of autologous BMSCs may have limited potential for providing starting material for cellular therapeutic applications.
One of the attractive advantages of BMSCs as a source of cell transplantation is their low immunogenicity. Recently, several studies have reported that BMSCs may be immune-privileged cells that do not elicit immune responses due to an absence of their immunologically relevant cell surface markers. BMSCs also are known to inhibit proliferation of T lymphocytes, B lymphocytes, dendritic cells, and natural killer cells [3, 14, 24, 26, 31]. This poses the intriguing possibility of utilizing allogenic or xenogenic BMSCs as an alternative in patients who have limited availability of autologous BMSCs. While allogenic BMSCs might be preferable to xenogenic ones, in humans, viable allogeneic tissue is not always readily available. Further, allogenic human cells have the potential for carrying disease, whereas the risk of such disease transmission might be minimized in genetically engineered animals that are specifically bred to serve as donors of BMSCs [2].
Based on these findings, the authors performed the current study to investigate whether the transplanted xenogenic BMSCs can survive and induce new bone formation in the posterolateral lumbar spine of the rabbit without using immunosuppressive medications. To our knowledge, this is the first study to report the feasibility of xenogenic BMSCs transplantation in a spine fusion model.
Materials and methods
BMSCs isolation, culture, and expansion in medium with osteoinduction supplement
The isolation, culture, and expansion of BMSCs in medium with osteoinduction supplement were performed according to previously published methods. Briefly, four Sprague–Dawley male rats (1-month old) were killed by pentobarbital overdose. Bone marrow plugs were obtained from bilateral femurs of male rats, flushed out using 10 mL of culture medium (alpha-modified Eagle’s medium, α-MEM) containing 10% inactivated FBS, antibiotics, l-ascorbic acid (50 μg/mL), sodium-glycerolphosphate (10 mmol/L) and dexamethasone (10 nmol/L), and expelled from a syringe through a 22-G needle. The released cells were collected in 25 T flask containing 5 mL of culture medium. The culture medium was changed after the first 24 h to remove nonadherent cells. Subsequently, the culture medium was changed three times a week. The cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C. The BMSCs of four or five passages were used for this experiment.
Implantation of BMSCs loaded onto CRM
A compression resistant matrix (CRM, Medtronic Sofamor Danek, USA) used in this study was chemically composed of 15% hydroxyapatite (HA) and 85% β-tricalcium phosphate (β-TCP) with 500 μm average pore size and 80% porosity [1]. Aliquots of BMSCs (1 × 106 cells) were incubated with 5 cc CRM matrix in 25 T culture flask for 2 h to allow attachment; 32 female New Zealand rabbits (3.5–4 kg) were divided into two groups in this study. In the experimental (xenogenic BMSCs + CRM) group, BMSCs loaded onto 5 cc CRM were implanted bilaterally onto the L4-5 intertransverse processes of 16 female rabbits. In the control (CRM alone) group, 16 female rabbits received 5 cc CRM alone at the L4-5 intertransverse processes. To exclude the possibility of migration of BMSCs from the transverse processes of the recipient female rabbits, we did not decorticate the transverse processes. No rabbits received any immunosuppressive medications during the experiment. Serial plain radiographs of the lumbar spine were obtained at 1, 2, 4, and 6 months post-operatively using a tube-to-plate distance of 90 cm. Four rabbits each in both the experimental and the control groups were killed at 1, 2, 4, and 6 months after implantation, and the lumbar spine underwent histological analysis. The lumbar spines (L3–L6) were harvested from the rabbits immediately after being killed. The specimens were fixed in 4% paraformaldehyde at 4°C for 48 h, decalcified in 20% EDTA (pH 7.4) for 6 weeks, dehydrated and divided into two segments longitudinally. One segment was embedded in paraffin for histological analysis, and another one was used for PCR for Sry gene (Y-chromosome-specific marker).
Polymerase chain reaction (PCR) for Sry gene (Y-chromosome- specific marker)
Genomic DNA was extracted from the lumbar spines of the rabbits using QIAamp DNA Micro Kit according to the manufacturer’s instructions. Surviving transplanted xenogenic BMSCs were identified by determining the expression of Sry gene, a Y-chromosome-specific DNA sequence, and a known autosomal control gene (glyceraldehydes-3-phosphate dehydrogenase, GAPDH). Primers for Sry gene (5′-TCAACAGAATCCCAGCAT-3′ and 5′-CCTTCGATGAGGCTGATA-3′) and GAPDH (5′-ATCATCTCCGCCCCTTCTGC-3′ and 5′-GCCTGC TTCACCACCTTCTT-3′) were synthesized on a DNA synthesizer. The PCR reaction mixture contained 1 μg of genomic DNA, 1.5 U of AmpliTaq, 20 pmol of rat Sry-specific primers, 10 pmol of rat GAPDH-specific primers. The PCR reaction was carried out in a programed thermal cycler for 35 cycles of denaturation (95°C for 1 min), annealing (48.3°C for 1 min), and extension (72°C for 1 min). Each PCR product was analyzed on a 2% agarose gel by electrophoresis. The gels were then stained with ethidium bromide and exposed to UV light.
Histological analysis
The paraffin block was sectioned 4-μm thick and dewaxed in xylene for 5 min and rehydrated through graded alcohol, stained with hematoxylin-eosin and Masson’s trichrome methods, respectively. Histological analyses to determine new bone formation and local inflammatory reactions were performed under light microscopy.
Results
All recipient female rabbits tolerated the surgical procedure well and were mobile the same day as of surgery. Serious complications related to the surgical procedure, such as death and nerve injury, were not observed. During the experiment, all surviving rabbits were observed to behave and feed normally. No post-operative deep wound infection was found in either group.
Survival of transplanted xenogenic BMSCs
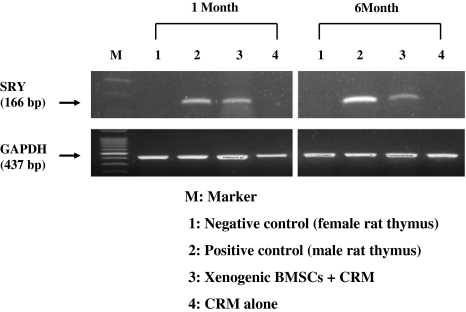
The expression of Sry gene, a Y-chromosome-specific marker of a male rat, was clearly identified in the lumbar spines of all 16 rabbits in the xenogenic BMSCs + CRM group at 1, 2, 4, and 6 months postimplantation, compared with those of CRM alone group (Fig. 1).
Fig. 1.
Polymerase chain reaction (PCR) showing the expression of Sry gene (a Y-chromosome-specific DNA sequence) in lumbar spines of xenogenic bone marrow stem cells (BMSCs) + compression resistant matrix (CRM) group at 1 and 6 months post-implantation, compared with those of CRM alone group
New bone formation of transplanted xenogenic BMSCs
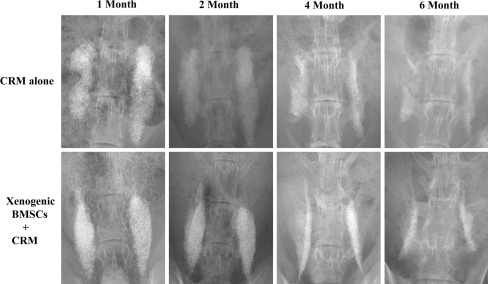
Serial plain radiographs demonstrated gradual resorption of CRM over time in both xenogenic BMSCs + CRM group and CRM alone group; however, it was difficult to clearly identify new bone formation due to the radiopacity of the remaining CRM at any follow-up point (Fig. 2).
Fig. 2.
Serial plain radiographs showed gradual absorption of CRM in both xenogenic BMSCs +CRM group and CRM alone groups. However, it was difficult to clearly identify new bone formation due to the radiopacity of the remaining CRM
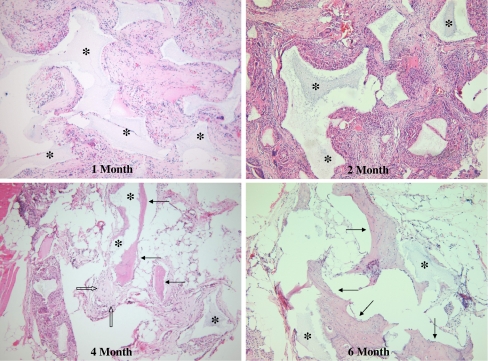
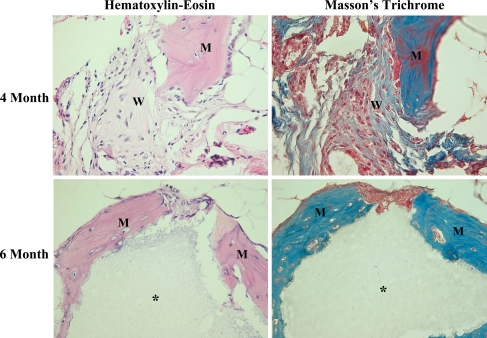
Histologically, mature lamellar and woven bone with osteoblasts and osteocytes were identified around the remaining CRM carriers in all eight rabbits in the xenogenic BMSCs + CRM group at 4 and 6 months after implantation, but in none of the eight rabbits at 1 and 2 months postimplantation (Fig. 3). The amount of newly formed bone was not sufficient to achieve solid fusion. None of CRM alone group showed evidence of new bone formation at 1, 2, 4 and 6 months postimplantation; only fibrous tissue was found around the remaining CRM carriers. Mild infiltration of inflammatory cells was identified around the remaining CRM carriers in both groups (Fig. 4).
Fig. 3.
Hematoxylin–eosin stain showing the presence of mature lamellar (arrows) and woven bone with osteoblasts (hollow arrows) around the remaining CRM carriers (asterisk) in xenogenic BMSCs +CRM group at 4 and 6 months post-implantation, but not at 1 and 2 months post-implantation (×100)
Fig. 4.
Hematoxylin–eosin and Masson’s trichrome stains more clearly showing the presence of mature lamellar (M) and woven bone (W) with osteoblasts were identified around the remaining CRM (asterisk) in xenogenic BMSCs + CRM group at 4 and 6 months post-implantation (×400)
Discussion
It has been reported that BMSCs may be immune-privileged cells that do not elicit immune responses due to an absence of immunologically relevant cell surface markers. In addition, BMSCs have immunomodulatory function [3, 14, 24, 25, 31]. Such immunological characteristics of BMSCs theoretically can make them impervious to immunorejection following xenogenic transplantation, irrespective of the use of immune suppression. Previous studies have reported conflicting results following xenogenic BMSCS transplantation into non-immunosuppressed hosts, ranging from no survival to differentiation into destination cells [9, 19, 28, 30]. However, in our literature review, we were unable to find any study that had investigated the results of xenogenic BMSCs transplantation in a spine fusion model. The authors therefore performed the current study to investigate the hypothesis that xenogenic BMSCs transplanted into the spine can survive and induce new bone formation without immune suppression.
To our knowledge, this is the first demonstration of the survival of transplanted xenogenic BMSCs in a posterolateral lumbar fusion model using PCR for Y-chromosome-specific DNA sequences (Sry gene). The accurate detection of surviving donor cells is critical for BMSCs transplantation models. Since the PCR can detect small amounts of gene-specific DNA sequences, this technique has recently been applied to investigate the survival of donor cells in sex-mismatched transplantation models (male donor, female host) [12, 20, 22, 29, 32]. Moreover, in the current study, we used a competitive PCR method, which detects both Sry and GAPDH genes, since a visible GAPDH band demonstrates a technically successful PCR reaction. Therefore, our results suggest that male rat BMSCs, which were xenogenically transplanted into the posterolateral lumbar spine of female rabbits, successfully survived without immune suppression.
The CRM has been demonstrated to be an effective osteoconductive material in previous spine fusion study [1]. We therefore used CRM as a carrier of xenogenic BMSCs in this study. The presence of callus material began to appear at 4 months and increased at 6 months postimplantation on serial plain radiographs. The CRM carriers were gradually resorbed over time (from 1 to 6 months after implantation) as expected. However, some CRM still remained. Therefore, it was difficult to clearly identify the newly formed bone on radiographs due to the radiopacity of the remaining CRM. Perhaps computed tomography might more precisely evaluate the presence of new bone formation in future studies. In the present study, our goal was to detect new bone formation, not solid fusion. We therefore did not perform manual palpation and biomechanical testing.
We found histological evidence of new bone formation in the xenogenic BMSCs + CRM group at 4 and 6 months after implantation. However, the amount of newly formed bone was small and not enough to achieve a solid fusion. One possible reason for the small amount of new bone formation might have been due to short incubation time (2 h) such that an insufficient number of xenogenic BMSCs were able to attach to the CRM carriers. Another possible reason is that the dose of xenogenic BMSCs (1 × 106 cells) used in this study may have been suboptimal. The optimal dose and incubation time remain to be determined in future studies that we are currently planning. Although mild infiltration of inflammatory cells was identified around the CRM carriers in both of experimental and control groups, no post-operative wound infection was found. Moreover, all animals were observed to behave and feed normally. Considering the expression of Sry gene and presence of new bone formation, transplanted xenogenic BMSCs were not rejected by immune system. After survival, xenogenic BMSCs differentiated into the osteoblasts and induced new bone formation in the posterolateral lumbar spine.
In conclusion, our results suggest that xenogenic BMSCs may provide an alternative to autologous BMSCs in situations where the host has limited availability of autologous BMSCs due to old age, osteoporosis, and metabolic diseases. This suggests the intriguing possibility of utilizing genetically engineered and specifically bred animals that are free of transmissible diseases as a source of BMSCs. To determine if a solid fusion can be achieved with such techniques, further studies are needed to investigate the appropriate dose of xenogenic BMSCs, amounts of CRM carriers, and the requisite incubation time.
Acknowledgment
This work was supported by year 2006 research fundings of Catholic Institute of Cell Therapy, The Catholic University of Korea School of Medicine.
References
- 1.Akamaru T, Duh D, Boden SD, Kim HS, Minamide A, Louis-Ugbo J. Simple carrier matrix modification can enhance delivery of recombinant huma bone morphogenic protein-2 for posterolateral spine fusion. Spine. 2003;28:429–434. doi: 10.1097/00007632-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Arinzeh TL, Peter SJ, Archambault MP, Bos C, Gordon S, Kraus K, et al. Allogenic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew A, Sturgeon C, Siatkas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocu\yte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 4.Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989;240:270–280. [PubMed] [Google Scholar]
- 5.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger M. Aging bone and osteoporosis. Strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237–2246. doi: 10.1001/archinte.163.18.2237. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 9.Grinnemo KH, Mansson A, Dellgren G, Klinberg D, Wardell E, Drvota V, et al. Xenoreativity and engraftment of human mesenchymal stem cells transplantation into infracted rat myocardium. J Thorac Cardiovasc Surg. 2004;27:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Heersche JN, Bellows CG, Ishida Y. The decerase in bone mass associated with aging and menopause. J Prosthet Dent. 1998;79:14–16. doi: 10.1016/S0022-3913(98)70187-8. [DOI] [PubMed] [Google Scholar]
- 11.Heise U, Osborn JF, Duwe F. Hydroxyapatite ceramic as a bone substitute. Int Orthop. 1990;14:329–338. doi: 10.1007/BF00178768. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa A, Tsujimoto G, Okuyama S, Li XK, Iwaya M, Masaki Y, et al. Polymerase chain reaction of the rat sex-determining region of the Y-chromosome and its application to estimate a state of sensitization to minor histocompatability antigen H-Y. Transplant Proc. 1995;27:1598–1600. [PubMed] [Google Scholar]
- 13.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 15.Le Heuc JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tri-calcium pjosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg. 1997;63:202–211. [PubMed] [Google Scholar]
- 16.Lennon DP, Haynesworth SE, Bruder SP, Jaiswal NJ, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol. 1996;32:602–611. doi: 10.1007/BF02724045. [DOI] [Google Scholar]
- 17.Lieberman JR, Daluiski A, Stevenson S, Wu L, McAlister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lou J, Xu F, Merkle K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald DJ, Luo J, Saito T, Duong M, Bernier PL, Chiu RC, et al. Persistence of marrow stromal cells implanted into acutely infarcted myocardium: observations in a xenotransplant model. J Thorac Cardiovasc Surg. 2005;130:1114–1121. doi: 10.1016/j.jtcvs.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu K, Valenzuela RG, Bishop AT. Detection of chimerism following vascularized bone allotransplantation by polymerase chain reaction using a Y-chromosome specific primer. J Orthop Res. 2003;21:1056–1062. doi: 10.1016/S0736-0266(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 21.Passuti N, Daculsi G, Rogez JM, Martin S, Bainvel JV. Macroporous calcium phosphate ceramic performance in human spine fusion. Clin Orthop Relat Res. 1989;248:169–176. [PubMed] [Google Scholar]
- 22.Patri S, Dascalescu C, Chomel JC, Sadoun A, Lacotte L, Tanzer J, et al. Monitoring and prognosctic evaluation of sex-mismatched bone marrow transplantation by competitive PCR on Y-chromosome sequences. Bone Marrow Transplant. 1996;17:625–632. [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1997;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Ringe J, Kaps C, Schmitt B, Büscher K, Bartel J, Smolian H, et al. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res. 2002;307:321–327. doi: 10.1007/s00441-002-0525-z. [DOI] [PubMed] [Google Scholar]
- 25.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Riew KD, Wright NM, Cheng S-L, Avioli LV, Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357–360. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 27.Russel JL, Block JE. Surgical harvesting of bone graft from the ilium: point of view. Med Hypotheses. 2000;55:474–479. doi: 10.1054/mehy.2000.1095. [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24. doi: 10.1016/S0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro H, Fukuda Y, Hoshino S, Furukawa M, Shintaku S, Dohi K. Monitoring for engraftment following rat orthotopic liver transplantation by in vitro amplication of Y-chromosome gene using polymerase chain reaction. Cell Transplant. 1995;4:61–63. doi: 10.1016/0963-6897(94)00060-W. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chen X, Armstrong MA, Li G. Survival of bone marrow-derived mesenchymal stem cells in a Xenotransplantation model. J Orthop Res. 2007;25:926–932. doi: 10.1002/jor.20385. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Lu XF, Lu YR, Liu J, Gao K, Zeng YZ, et al. Immunogenicity and immune modulation of osteogenic differentiated mesenchymal stem cells from Banna Minipig Inbred Line. Transplant Proc. 2006;38:2267–2269. doi: 10.1016/j.transproceed.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Wilborn F, Schmidt CA, Siegert W. Demonstration of chimerism after allogenic bone marrow transplantation by polymerase chain reaction of Y-chromosome-specific nucleotide sequences—characterization of a technical approach. Leukemia. 1993;7:140–143. [PubMed] [Google Scholar]