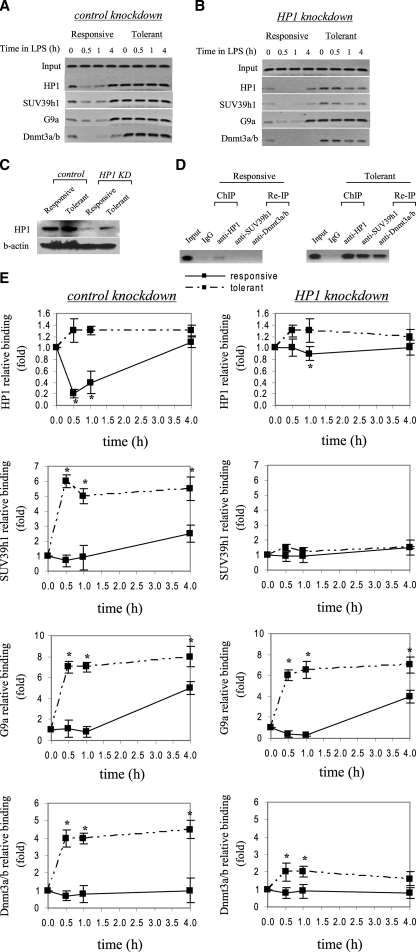
FIGURE 3.
HP1 gene silencing markedly reduces SUV39h1, Dnmt3a/b, but not G9a binding to the TNFα promoter. THP-1 cells were transfected with control or HP1-specific siRNA and then left unstimulated or stimulated with 1 μg/ml LPS for 36 h. Cells were then washed and left unstimulated or stimulated with 1 μg/ml LPS for the indicated times. ChIP assay was performed on cross-linked chromatin, immunoprecipitated with antibodies specific to HP1, SUV39h1, G9a, and Dnmt3a/b. The relative enrichment of TNFα promoter sequences in the immunoprecipitated DNA was analyzed by semiquantitative PCR (A and B) and real-time PCR (E) using primers that amplify the proximal promoter region. In A and B, representative results are shown. In E, data were normalized to input DNA and are presented as fold change relative to unstimulated cells (0 h) (set as 1-fold). Data are the mean ± S.E. of at least three independent experiments. *, significant difference (p < 0.05). C, HP1 protein expression in the nucleus. THP-1 cells were transfected with control or HP1-specific siRNA and left unstimulated or stimulated with 1 μg/ml LPS for 36 h. Responsive (unstimulated) and tolerant (stimulated) cells were then washed and stimulated for 1 h with 1 μg/ml LPS, and nuclear extract was prepared as described under “Experimental Procedures.” Blotted proteins were probed with HP1 Ab. Blots were stripped and reprobed with control Ab. D, ChIP reimmunoprecipitation showing the recruitment of SUV39h1 and Dnmt3a/b to the TNFα promoter by HP1. Chromatin was first immunoprecipitated with HP1 Ab (ChIP). Immunoprecipitates were then eluted and reimmunoprecipitated (Re-IP) with SUV39h1 or Dnmt3a/b Ab.