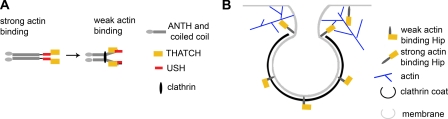
FIGURE 8.
Model of Hip protein conformations and effect on vesicle budding. A, model of the mechanism for regulation of actin binding to Hip proteins by clathrin light chain. Flexibility in the coiled-coil allows large scale bending of the coiled-coil upon clathrin light chain binding. This could reduce actin binding by the THATCH domain by direct blocking resulting from an intramolecular interaction between THATCH and a site that is exposed following clathrin light chain binding or indirectly by light chain inducing a conformational change that repositions the USH to an inhibitory conformation or a combination of both mechanisms. B, the decreased affinity of Hip1 and Hip1R for actin while bound to clathrin light chain suggests that Hip proteins do not interact with actin while incorporated into the clathrin coat. Instead, Hip proteins may interact with actin at the neck of the budding vesicle or edge of the clathrin coat, promoting development of a budding vesicle.