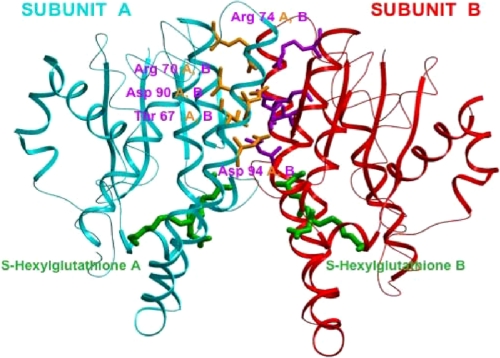
FIGURE 1.
Dimeric structure (side view) of human GST π (PDB 9GSS) crystallized with S-hexylglutathione, which is colored green. This structure shows the electrostatic region between two subunits. Each individual subunit is colored-coded: the backbone of subunit A is shown in cyan, while that of subunit B is red. The residues selected for mutagenesis at the subunit interface are Arg-70, Arg-74, Asp-90, Asp-94, and Thr-67. The side chains of the A subunit amino acid residues are in yellow, whereas those of the B subunit are purple.