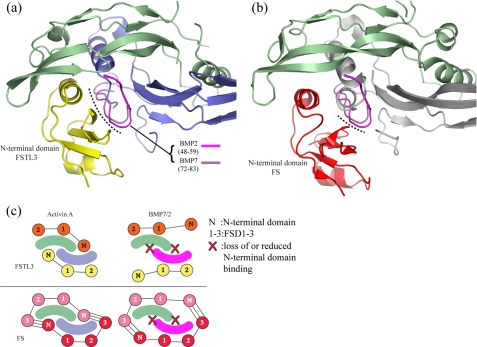
FIGURE 7.
Structural differences between activin A and BMP may explain antagonist
specificity. a and b, superposition of BMP2 and BMP7
onto the FSTL3·activin A complex, aligning only monomer 1 of activin A.
The ribbon of activin A is shown, but for clarity, only the prehelix loop
residues for each BMP (BMP2 (pink) and BMP7 (purple)) are
depicted. The dotted lines indicate regions on BMP close enough to
clash with the N-terminal domains. These appear more extensive for FSTL3.
c, overall scheme for FSTL3 and FS binding to activin A and BMPs. We
propose that the N-terminal domain of FS-type antagonists does not interact
favorably with BMPs, thus accounting for the decreased affinity. We propose
that the low affinity still observed for FS may be a result of the
FS(ND) FS(FSD3) interactions
and/or structural variation in the antagonist N-terminal domains.
FS(FSD3) interactions
and/or structural variation in the antagonist N-terminal domains.