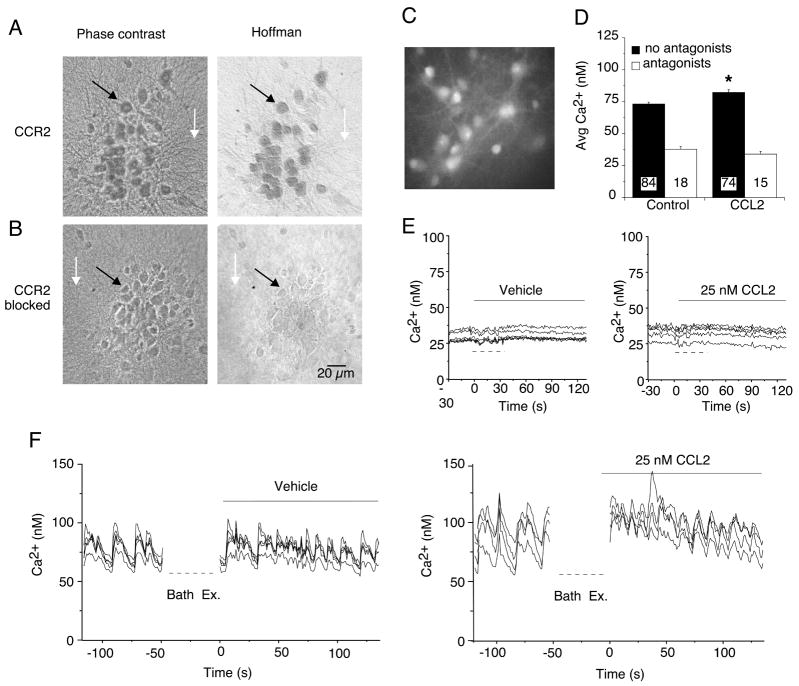
Figure 4.
Hippocampal neurons express functional CCR2. (A) Immunocytochemical staining of rat hippocampal cultures using the antibody specific against CCR2B (1:100 dilution). Prominent immunostaining was observed in the neurons (e.g., black arrow) but not in the underlying glial cell layer (e.g., white arrow). Neurons were identified by morphological criteria established by immunostaining with cell type specific antibodies. The phase contrast images (left panel) show all cells in the microscopic field. The Hoffman optics images (right panel) show the immunostained cells in the field. (B) Specific immunostaining for CCR2 was blocked by preincubation of the antibody with the antigenic peptide used to raise the CCR2 antibody. (C–F) Effect of CCL2 on Ca2+ signals generated by spontaneous network synaptic activity in hippocampal neurons. (C) Gray scale digitized image showing a representative field of fura-2 loaded hippocampal neurons. (D) Mean (SEM) values for average Ca2+ levels under control conditions and after addition of CCL2 (25 nM) to the bath saline. Recordings were made in the presence (antagonists) and absence (no antagonists) of TTX plus glutamate and GABA receptor antagonists. Numbers in bars indicate the number of cells measured. (E, F) Representative recordings of intracellular Ca2+ levels before and after (solid bars) bath exchange. Dashed lines below the traces indicate when the bath was exchanged during the recording. In F, the recording was stopped during the bath exchange. In E, the bath contained TTX plus receptor antagonists to block synaptic network activity. In F, TTX and receptor antagonists were not used. The vehicle control bath exchange was used to identify potential changes in Ca2+ signals due to mechanical stimuli caused by the bath exchange.