Figure 1. Summary of paraquat redox cycling and detoxification of ROI by cellular antioxidants.
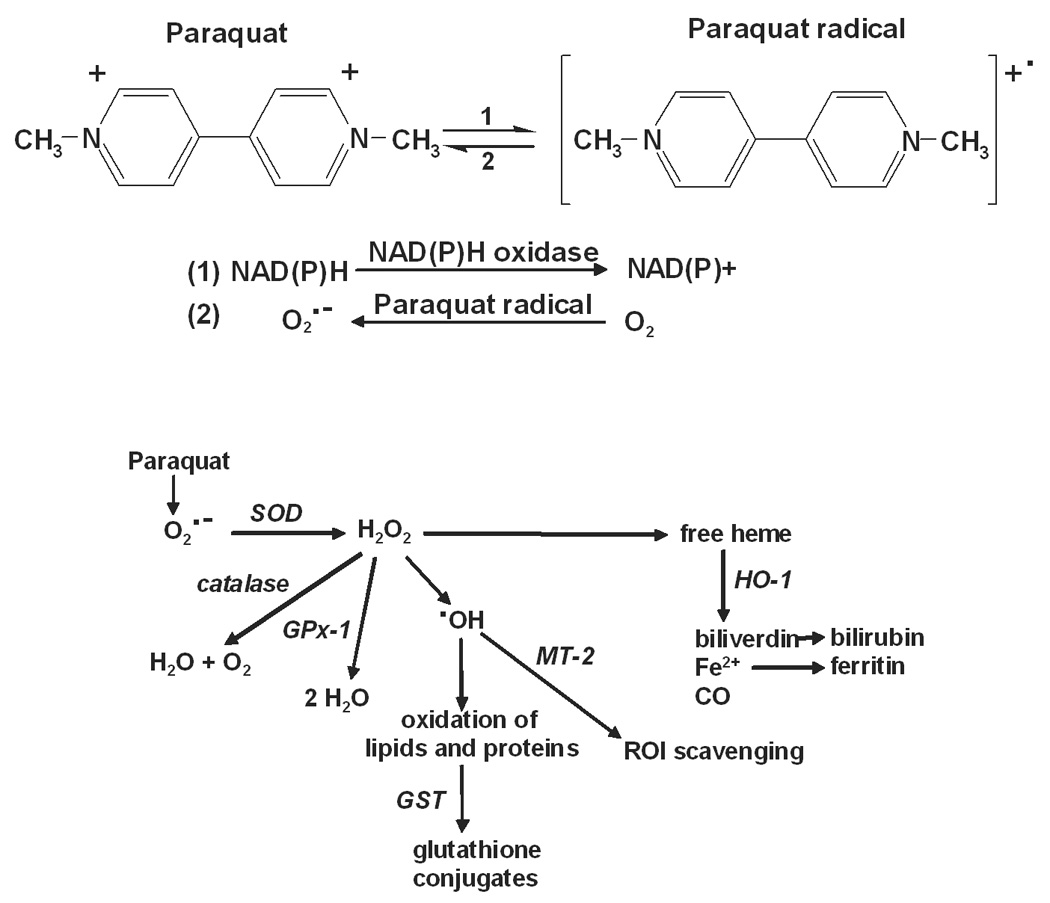
Upper panel: Paraquat redox cycling reactions. Paraquat undergoes a one electron reduction to the paraquat radical via the oxidation of NAD(P)H to NAD(P)+ by NAD(P)H oxidase (Reaction 1). The paraquat radical is then immediately oxidized to the parent compound with the transfer of an electron to molecular oxygen, forming superoxide anion (Reaction 2). Lower panel: Detoxification of ROI by enzymatic antioxidants. Superoxide anion is metabolized to hydrogen peroxide by SOD. Hydrogen peroxide is detoxified by catalase and/or various peroxidases including GPx-1. Hydrogen peroxide has been implicated in the mobilization of heme, an oxidizing agent, which is then degraded by heme oxygenases including HO-1 to biliverdin, ferric iron and carbon monoxide. Biliverdin is further metabolized to bilirubin and ferric iron is removed through sequestration by ferritin. In the presence of transition metals such as iron or copper, hydrogen peroxide can also be converted to hydroxyl radicals. The zinc-mediated free radical scavenging function of MT-2 serves as a mechanism for removal of hydroxyl radicals. Oxidized proteins and lipids can be conjugated with glutathione by the GST enzymes to facilitate cellular elimination.
