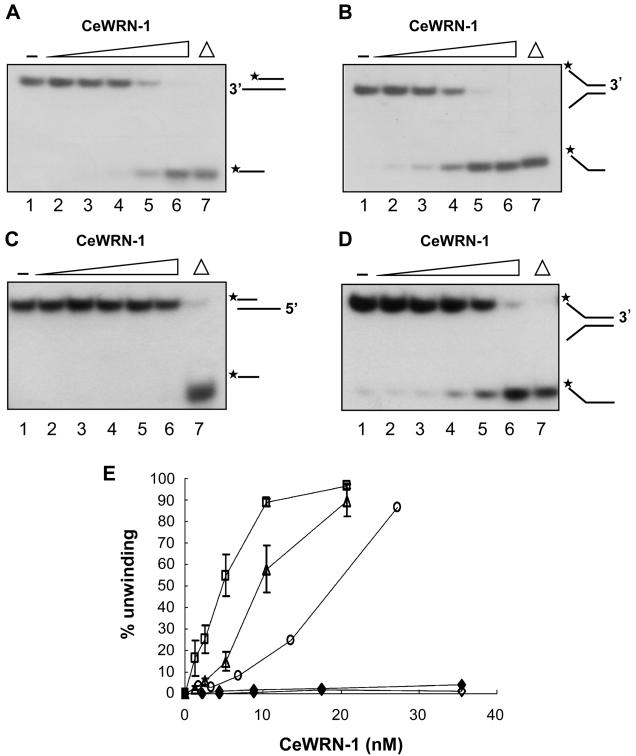
Figure 2. Helicase activity and polarity of CeWRN-1.
Substrates were (A) the 3′ ssDNA tailed duplex, (B) a short forked duplex, (C) the 5′ ssDNA tailed duplex, and (D) a 34 bp forked duplex. Helicase reactions were carried out as described in Materials and Methods: lane 1, no enzyme control; lane 7, heat-denatured DNA substrate. Lanes 2–6 show a protein titration (1.5, 3.0, 6.0, 12, and 24 nM for panels A and B; 2.2, 4.4, 8.8, 17.6, and 35.2 nM for panel C; and 1.7, 3.4, 6.8, 13.6, and 27.2 nM for panel D). The substrates and products are shown at the right. The asterisk denotes the 32P label. (E) Quantitation of the data from panels A–D and Figure S1 of the Supporting Information. The percentage unwinding is expressed as described in Materials and Methods. Data represent the means of at least three independent experiments ± SD: (□) a 22 bp fork, (△) the 3′ ssDNA tailed duplex, (◆) the 5′ ssDNA tail, (◇) the blunt-ended duplex, and (○) a 34 bp fork.