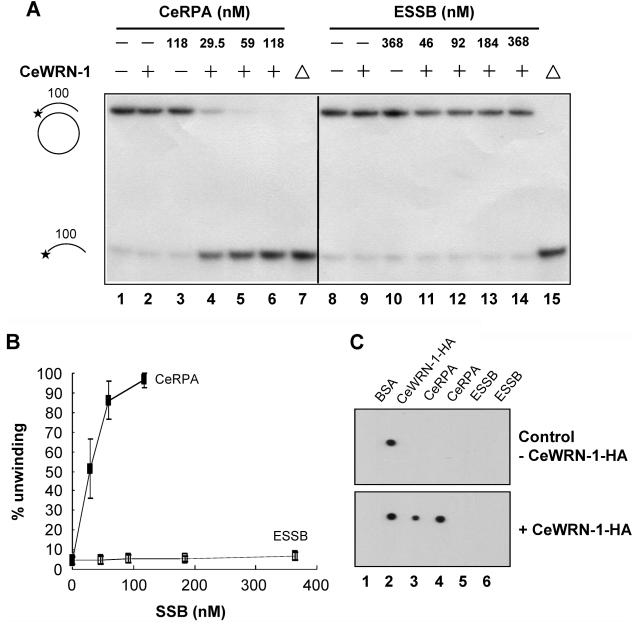
Figure 7. Stimulation of CeWRN-1 helicase activity on a 100 bp partial DNA duplex substrate.
(A) CeWRN-1 protein (40 nM) was incubated with the 100 bp partial duplex in the presence of the indicated concentrations of CeRPA or ESSB protein under the standard helicase reaction conditions as described in Materials and Methods: lanes 1 and 8, no enzyme control; lanes 2 and 9, CeWRN-1 control; lane 3, CeRPA control; lane 10, ESSB protein control; lanes 7 and 15, heat-denatured DNA substrate control; lanes 4–6, a protein titration (CeRPA at 29.5, 59, and 118 nM, respectively); and lanes 11–14, a protein titration (ESSB at 46, 92, 184, and 368 nM, respectively). (B) Quantitation of results presented in panel A: (■) CeRPA and (□) ESSB protein. Percentage displacement is expressed as a function of SSB protein concentration. Data represent the means of at least three independent experiments ± SD. (C) Dot blot assay. BSA, CeWRN-1, CeRPA, and ESSB as indicated were immobilized on PVDF membranes. The membranes were blocked for 2 h at 4 °C and incubated in the absence or presence of CeWRN-1 as indicated at 4 °C overnight. After being washed, the membranes were incubated with monoclonal anti-HA antibody. Western blotting was performed as described in Materials and Methods.