Table 3.
Carbonylation of fluorinated epoxides using 1 at 100 psi CO.
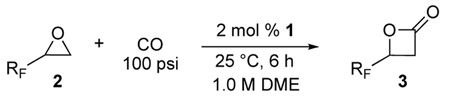 | |||||
|---|---|---|---|---|---|
| Entry | Epoxide | β-Lactone | Conv. (% Yield)a | ||
| 1 | 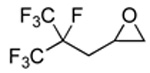 |
2a | 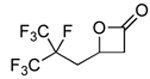 |
3a | 99 (86) |
| 2 | 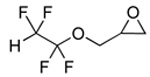 |
2b |  |
3b | 99 (79) |
| 3 |  |
2c |  |
3c | 99 (85) |
| 4 |  |
2d |  |
3d | 99 (88) |
| 5 |  |
2e |  |
3e | 99 (91) |
| 6 | 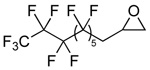 |
2f |  |
3f | 99 (71) |
| 7 | 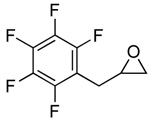 |
2g | 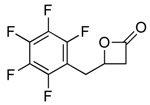 |
3g | 99 (82) |
β-Lactone was the sole product as determined by 1H NMR spectroscopy; % yield of isolated, analytically pure material.
