Abstract
Optical density measurements were used to estimate the effect of heat treatments on the single-cell lag times of Listeria innocua fitted to a shifted gamma distribution. The single-cell lag time was subdivided into repair time (the shift of the distribution assumed to be uniform for all cells) and adjustment time (varying randomly from cell to cell). After heat treatments in which all of the cells recovered (sublethal), the repair time and the mean and the variance of the single-cell adjustment time increased with the severity of the treatment. When the heat treatments resulted in a loss of viability (lethal), the repair time of the survivors increased with the decimal reduction of the cell numbers independently of the temperature, while the mean and variance of the single-cell adjustment times remained the same irrespective of the heat treatment. Based on these observations and modeling of the effect of time and temperature of the heat treatment, we propose that the severity of a heat treatment can be characterized by the repair time of the cells whether the heat treatment is lethal or not, an extension of the F value concept for sublethal heat treatments. In addition, the repair time could be interpreted as the extent or degree of injury with a multiple-hit lethality model. Another implication of these results is that the distribution of the time for cells to reach unacceptable numbers in food is not affected by the time-temperature combination resulting in a given decimal reduction.
Heat treatment is a method of preservation widely used in the food industry (24). Relatively mild thermal treatments below 100°C are sufficient to kill vegetative cells of food-borne pathogens or spoilage organisms, while inactivation of bacterial spores in nonacid foods requires much higher process temperatures, typically 121°C or more. In early studies to develop processes for the safe production of canned foods, the concept of thermal death point was used, this being defined as the length of time at different temperatures needed to destroy a definite concentration of spores under defined conditions (3). The work of Esty and Meyer (8) demonstrated a linear relationship between heating temperature and the logarithm of the time needed to inactivate suspensions containing 60 billion spores of proteolytic Clostridium botulinum. This work, often cited as the first example of a predictive model, led to the standard for canned food sterilization being based on a reduction of the spore population by a factor of 1012.
Studies on the kinetics of thermal inactivation established that the concentration of viable vegetative cells or spores decreases more or less exponentially with time of heating such that a plot of the logarithm of the viable cell concentration versus time yields a straight line (3, 6). The assumption of a logarithmic order of death underlies thermal process calculations based on D and z values, where D is the time needed for a 10-fold reduction in viable numbers and z is the temperature change needed to bring about a 10-fold change in D. While this has served the food industry very well, many researchers have reported deviations from log linearity, particularly in the case of vegetative cells, and “shoulders” and “tails” on semilogarithmic survival curves are often observed (22, 23).
The log-linear relationship for thermal inactivation is most commonly interpreted according to the thermodynamic principles developed by Rahn (26) and more recently by McKee and Gould (17). According to them, death of a cell occurs when a critical target molecule is inactivated by interaction with water or other surrounding molecules. These theories are simple but rely on numerous assumptions that are disputable. Many alternative “hit theories” have been developed. In particular, the conceptual model for sublethal injury introduced by Gould (13) proposes that heat simultaneously causes destruction of critical targets and many other cell components that are present in higher copy numbers. These secondary targets are less critical unless reduced to very low levels or the cell is stressed during recovery.
A large number of cell components are affected by exposure to elevated temperatures, and it is difficult to identify a critical target whose loss leads to cell death. The most likely candidates are DNA, ribosomes and RNA, the cytoplasmic membrane, and particular enzymes (13). Whether or not cellular damage leads to cell death depends on the extent of injury and also on recovery conditions after exposure to stress. Resynthesis of 30S ribosomes is possible after mild heat stress, as is restoration of outer membrane integrity in heat-injured gram-negative bacteria (16, 29). Incubation under anaerobic conditions can circumvent oxidative damage and allow cells to recover resistance to aerobic conditions (5, 12). Repair of sublethal injury requires biosynthesis to restore lost components, and this causes a delay before cell division becomes possible.
One way of measuring the effect of the stress of heat treatment on the cells is by measuring the lag times of the single cells which recover. It has indeed been shown that lag times can vary widely between individual cells in a population, and the inherent variability in the lag time of single cells increases with severity of heat treatment (7, 15, 27, 28). Knowing how heat treatments affect the variability of single-cell lag times is extremely important in assessing the risk of cell recovery and growth in processed foods where low numbers of stressed cells of pathogenic bacteria may be distributed among different packs of food. In terms of food safety, it would be desirable to predict what proportion of cells have very long or very short lag times and why and to integrate this knowledge in predictive models. Niven et al. (21) examined the effect of sublethal heating at 50°C on the distribution of single-cell lag times of Escherichia coli, but there have been no investigations of how the mean and spread of the distribution vary depending on the temperature and duration of the preceding heat treatment. This work therefore set out to examine how the parameters of single-cell lag time distributions of Listeria cells exposed to heat treatments of different severities (sublethal and lethal) vary as demonstrated by their lag time. A sublethal heat treatment refers to a treatment in which no loss of viability occurred and all cells were able to grow within 2 weeks. A lethal heat treatment refers to a treatment resulting in a measurable proportion of the cells being unable to grow within 2 weeks; the lag time measured is the lag time of the single cells which are able to grow, the survivors. We used optical density (OD) measurements to measure the time at which the populations grown from about one cell reached a detectable level. The recovery was under the same conditions for all heat treatments to avoid the effect of the medium on recovery.
Predictive microbiology models are commonly divided into primary and secondary models (30). The primary model describes the time variation of the microbial response to a given environment; the secondary model describes how the parameters of the primary model depend on the environmental factors. In this paper, we use a similar approach but instead of the time variation of a microbial response we describe the cell-to-cell variation of the lag times of single cells by a primary model and model the effect of the severity of the heat treatment (instead of the growth environment) on the parameters of the primary model.
MATERIALS AND METHODS
Culture preparation.
L. innocua strain NCTC 11288 (serotype 6a) was subcultured from stock slopes (stored at 3°C on tryptone soy agar [Oxoid, Basingstoke, United Kingdom]) to tryptone soy broth (Oxoid) and incubated at 30°C for 24 h. The culture was diluted 1:1,000 in maximum recovery diluent (Oxoid) and inoculated into 4× 30 ml TSYGB (tryptone soy broth plus 0.3% yeast extract plus 1% glucose), pH 7.0, to give approximately 1,000 cells/ml. The culture was then incubated statically at 22°C for 48 h to stationary phase.
Thermal inactivation curves.
Stationary-phase cultures were centrifuged (3,100 × g for 15 min at 4°C), and the pellets were combined and resuspended in a total volume of 2 ml TSYGB. This cell suspension was refrigerated until required but used within 2 h. Tubes with a rubber septum, containing 10 ml TSYGB, were submerged in a water bath to preheat to the required temperature. Tubes were vented with a sterile needle to release pressure and then injected with 100 μl cell suspension directly into the liquid using a precision syringe fitted with a long sterile needle. After the appropriate heating time, the tubes were removed from the bath and cooled rapidly in ice water. Cooled tubes were refrigerated until ready to enumerate survivors. Heated samples were serially diluted in TSYGB, and appropriate dilutions were inoculated into five tubes of TSYGB for enumeration by most probable number (MPN). Tubes were incubated at 22°C, and positives were recorded after 2 weeks. The log MPN of survivors was plotted against heating time to give thermal inactivation curves for each of the test temperatures.
Growth curve at 22°C.
Samples were taken from the cultures described above approximately hourly, diluted (serial 10-fold dilutions) in maximum recovery diluent, and plated onto triplicate tryptone soy agar plates. Plates were incubated at 30°C for up to 2 days. The logarithm of CFU ml−1 was plotted against time to determine the growth rate of the culture.
OD measurements.
Heated cell suspensions were diluted in TSYGB according to the severity of the heat treatment. The dilution necessary to obtain 20 viable cells ml−1 of diluent was estimated from the thermal inactivation curves. This was then used to inoculate wells of two microtiter plates at 50 μl per well to give on average one viable cell well−1. Growth medium (350 μl TSYGB, pH 7.0) was added to each well. Plates were incubated in the Bioscreen C (Labsystems, Finland) at 22°C, OD measurements at 600 nm (OD600) were taken at intervals for up to 2 weeks, again depending on the severity of the treatment. The detection time for each well, Tdet, (the time at which the OD reached 0.11), was calculated. An OD of 0.11 corresponded to a cell concentration of ydet = 107.7 CFU ml−1 as determined with a calibration curve (19).
Estimation of specific growth rate.
The specific growth rate, μ, in the microwell plates under the conditions described above was assumed to be the same in each well and in all experiments (19). The plate count growth curve (log CFU ml−1 versus time) was fitted to the model of Baranyi and Roberts (2) by the DmFit program (www.ifr.ac.uk/Safety/DMfit/default.html).
Interpretation and modeling of single-cell lag time.
The lag time of a single cell, Lg, was defined from the biphasic function
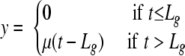 |
(1) |
where y is the natural logarithm of the single-cell-generated subpopulation (18; J. Baranyi, S. M. George and Z. Kutalik, submitted for publication). It could be checked experimentally that the population is still in the exponential phase at the detection level corresponding to the observation time, Tdet.
Since after a heat treatment, the cells need time for the biosynthesis of damaged components during which the cells are not able to divide, the lag time, Lg was divided into two components: a repair time, τr, and an adjustment time, τa. The former is the time the cells need to repair the damage caused by the heat treatment; the latter is the time they need to adjust to the new environment. When the cells are not stressed, then τr = 0 and the adjustment time is the same as the lag time. We assumed that Lg follows a shifted gamma distribution (18; Baranyi et al., submitted). With this assumption, τr (the shift in the distribution) is the same for all of the cells while τa varies randomly according to the probability distribution function, f(t), of the gamma distribution:
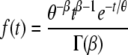 |
The parameters β and θ are the shape and scale parameters, respectively, and Γ(β) is the gamma function of β. The mean of the distribution is βθ, and its variance is βθ2.
With this repartition, all of the cell-to-cell variability is allocated to the adjustment time. In reality, τr is probably also variable, but it would be impossible to separate the different sources of variability. Both τr and τa depend on the heat treatment and the growth environment.
When many cells are grown together, the lag time for a population of N initial cells is smaller than τr + βθ, the repair time plus the expected value of the distribution of single-cell lag times, since the fast-growing subpopulations contribute more to the culture than the slow ones. However, as N→ ∞, it is possible to estimate the population lag time, λ, from the single-cell parameters (1):
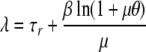 |
(2) |
Using the terminology discussed in the introduction, the primary model is the shifted gamma distribution of the single-cell lag times, characterized by the parameters τr, β, and θ. The secondary level is the modeling of the effect of the severity of the heat treatment, quantified by the heating temperature, Theat, and heating time, theat, on the parameters of the primary model.
Numerical estimations.
We assumed that the cell concentration at the detection level is the same in all of the wells. The growth rate was assumed to be the rate estimated by plate counts in all experiments. The initial number of cells in a well, N, follows a truncated Poisson distribution (18) with average
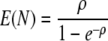 |
where ρ is the average of the Poisson distribution of the number of cells per well which was estimated from the number of empty wells.
With these assumptions, it has been shown that the parameters of the gamma distribution of the single-cell lag times can be estimated from the detection times using the three moments of the gamma distribution (Baranyi et al., submitted). The system of three nonlinear equations for τr, β, and θ was solved by numerical approximation with the nlreg program (www.nlreg.com).
The link function “natural logarithm” was used to characterize the single-cell lag time distribution parameters, while the decimal logarithm was used for the reduction in cell numbers and for the heating time as this is how heat treatments are traditionally modeled. The nonlinear regressions were carried out in Microsoft Excel, using its regression functions and the Solver add-in.
RESULTS
Growth rate.
The growth rate estimated from the plate count experiment fitted to the model of Baranyi and Roberts was 0.26 log CFU ml−1 h−1 (R2 = 0.99). This compares reasonably with the growth rate of 0.31 log CFU ml−1 h−1 obtained with the Growth Predictor software (www.combase.cc) for Listeria at pH 7 in 0.5% NaCl at 22°C.
Thermal inactivation.
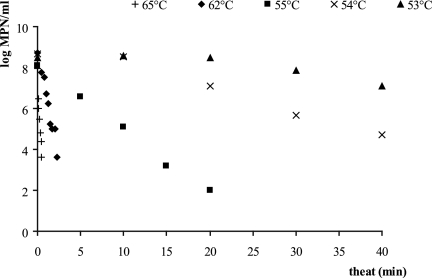
At 50°C and 52°C, no reductions in cell numbers were observed for heat treatments of up to 40 min. The thermal inactivation curves obtained at 53°C to 65°C for treatments up to 40 min are shown in Fig. 1. The decimal reduction in cell numbers as a function of the heating times was approximately linear for all temperatures tested. The values of the survival rate and the coefficients of determination at each temperature are given in Table 1.
FIG. 1.
Thermal inactivation curves of Listeria innocua heated in TSYGB.
TABLE 1.
Slope and coefficient of determinations of the linear regression of the thermal inactivation curves
| Temp (°C) | Inactivation rate (log MPN/ml/min) | Coefficient of determination |
|---|---|---|
| 53 | −0.034 | 0.752 |
| 54 | −0.107 | 0.956 |
| 55 | −0.312 | 0.996 |
| 62 | −2.195 | 0.972 |
| 65 | −7.899 | 0.968 |
The lethal heat treatments were those which lasted more than 20 min at 53°C, 10 min at 54°C, or 4 min at 55°C and all heat treatments carried out at 62 and 65°C. The other conditions were sublethal.
Primary model.
The minimum of the detection times in the Bioscreen increased with the length and temperature of the heat treatment (results not shown). As the recovery was carried out under the same controlled conditions for all experiments, it means that the time the cells needed to recover, τr, increased with the severity of the heat treatment. A measurable repair time, τr, was observed even after the mildest heat treatments. The parameters of the gamma distribution, τr, β, and θ, are given in Table 2 as well as the lethality of the heat treatment. The shape parameter of the adjustment time did not vary much from one experiment to another: the average value of β was 2.26 with a standard deviation of 0.57. In what follows, we assume it to be constant so only one parameter of the gamma distribution of τa remains to be fitted as a function of the severity of the heat treatments. This is equivalent to assuming that the coefficient of variation (CV), the standard deviation divided by the expected value of the adjustment time, was constant (in the case of a gamma distribution, 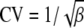 ). The CV was equal to 0.68 (average) with a standard deviation of 0.08.
). The CV was equal to 0.68 (average) with a standard deviation of 0.08.
TABLE 2.
Parameters of the shifted gamma distribution of the single-cell lag timesa
| Theat (°C) | theat (min) | W/Wtot | E(N) | τr (h) | β | θ (h) | Decimal reduction |
|---|---|---|---|---|---|---|---|
| 50 | 1 | 97/200 | 1.37 | 0.52 | 2.61 | 0.68 | 0 |
| 5 | 154/200 | 1.91 | −1.27 | 2.51 | 2.47 | 0 | |
| 20 | 164/199 | 2.11 | 5.29 | 2.30 | 1.67 | 0 | |
| 30 | 131/199 | 1.63 | 3.93 | 1.95 | 3.15 | 0 | |
| 40 | 148/199 | 1.83 | 5.52 | 2.02 | 4.39 | 0 | |
| 52 | 1 | 95/200 | 1.36 | 1.03 | 3.11 | 0.83 | 0 |
| 5 | 150/199 | 1.86 | 4.79 | 2.27 | 1.83 | 0 | |
| 10 | 153/200 | 1.89 | 9.44 | 2.08 | 4.82 | 0 | |
| 20 | 127/200 | 1.59 | 6.98 | 2.59 | 9.69 | 0 | |
| 30 | 75/200 | 1.25 | 12.10 | 1.70 | 14.27 | 0 | |
| 40 | 122/200 | 1.54 | 9.14 | 2.02 | 14.92 | 0 | |
| 53 | 2 | 158/199 | 1.99 | 2.22 | 3.47 | 1.22 | 0 |
| 4 | 170/199 | 2.25 | 6.26 | 2.84 | 2.09 | 0 | |
| 6 | 168/199 | 2.20 | 6.18 | 2.61 | 4.31 | 0 | |
| 8 | 153/199 | 1.91 | 9.26 | 2.20 | 5.73 | 0 | |
| 10 | 152/199 | 1.89 | 8.84 | 2.05 | 7.00 | 0 | |
| 20 | 173/199 | 2.34 | 9.90 | 2.48 | 17.21 | 0 | |
| 30 | 189/198 | 3.24 | 17.00 | 2.53 | 18.23 | 0.5 | |
| 40 | 164/199 | 2.11 | 20.90 | 2.35 | 21.85 | 1 | |
| 54 | 2 | 155/198 | 1.95 | 3.18 | 3.41 | 1.17 | 0 |
| 4 | 174/196 | 2.46 | 4.15 | 3.54 | 3.67 | 0 | |
| 6 | 138/198 | 1.71 | 13.00 | 1.94 | 9.26 | 0 | |
| 8 | 157/198 | 1.99 | 11.30 | 1.96 | 12.57 | 0 | |
| 10 | 129/196 | 1.63 | 16.30 | 1.96 | 15.71 | 0 | |
| 20 | 195/199 | 3.99 | 13.30 | 3.81 | 13.47 | 1 | |
| 30 | 128/195 | 1.63 | 31.00 | 1.77 | 30.29 | 2 | |
| 40 | 61/199 | 1.19 | 26.40 | 1.54 | 42.23 | 3 | |
| 55 | 4 | 126/199 | 1.58 | 8.77 | 1.82 | 7.49 | 0 |
| 8 | 131/199 | 1.63 | 20.70 | 1.83 | 43.54 | 0.5 | |
| 12 | 137/199 | 1.69 | 29.00 | 2.11 | 21.41 | 1.5 | |
| 18 | 169/199 | 2.23 | 26.80 | 1.87 | 62.23 | 2 | |
| 25 | 156/185 | 2.20 | 42.10 | 1.65 | 46.55 | 3 | |
| 30 | 123/195 | 1.58 | 61.50 | 1.64 | 52.08 | 4 | |
| 62 | 1 | 161/198 | 2.06 | 28.86 | 1.62 | 48.14 | 2 |
| 1.32 | 165/196 | 2.19 | 45.32 | 1.80 | 56.83 | 3 | |
| 1.75 | 181/200 | 2.60 | 41.09 | 2.12 | 42.82 | 4 | |
| 65 | 0.25 | 183/200 | 2.69 | 31.86 | 2.09 | 48.36 | 2 |
| 0.33 | 176/200 | 2.41 | 41.25 | 1.87 | 55.74 | 3 |
W is the number of wells showing growth, Wtot is the total number of wells taken into account in each experiment, E(N) is the average number of cells per well estimated from the number of empty wells (from a truncated Poisson distribution), τr is the repair time or shift of the distribution, and β and θ are the shape and scale parameters, respectively. The result of the heat treatment for 5 min at 50°C was not taken into account in the secondary modeling because of the negative shift.
Secondary models. (i) Repair time, τr.
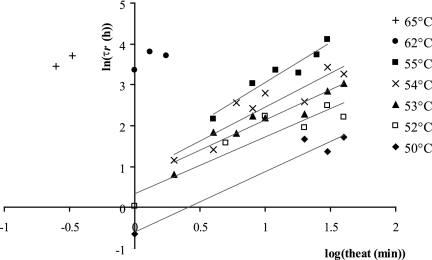
The natural logarithm of the repair time (h) as a function of the decimal logarithm of the heating time (min) is shown in Fig. 2 for the different temperatures. The curves are linear with coefficients of determination between 0.84 and 0.96 for the temperatures ranging from 50 to 55°C. It was shown by an analysis of the covariance that their slopes do not differ significantly (P = 0.92) and the common slope is 1.53 (including the experiments at 62 and 65°C). The intercepts too were a linear function of the heating temperature: intercept = 0.327 Theat − 16.76 (R2 = 0.99).
FIG. 2.
Secondary modeling: natural logarithm of the repair time, τr, as a function of the decimal logarithm of the heating time for the different temperatures. It can be shown statistically that the slopes are homogeneous.
So the repair time, τr, could be modeled with the equation
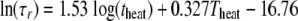 |
(3) |
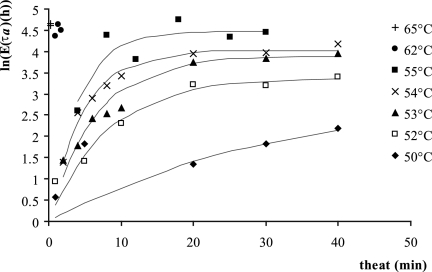
(ii) Mean of the adjustment time, τa.
As only one parameter of the gamma distribution needs to be modeled, we chose the mean, the standard deviation being proportional to it. The natural logarithm of the mean of the adjustment period, as a function of the heating time for the different temperatures, is shown in Fig. 3. It increased with the duration of the heat treatment but stabilized as the heat treatment became lethal. This was fitted with the asymptotic model often used for the product of first-order chemical reactions in batch:
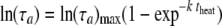 |
(4) |
where k and ln(τa)max are, respectively, a rate (min−1) and the maximum for the logarithm of the mean of τa (h) depending on the temperature.
FIG. 3.
Secondary modeling. The logarithm of the mean of the adjustment period, τa, was fitted to the equation 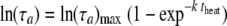 .
.
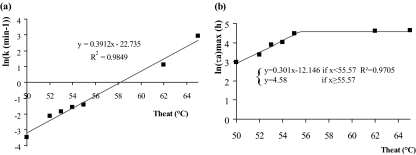
In Fig. 4, the logarithm of the rate, k (Fig. 4a), and the logarithm of the maximum, ln(τa)max (Fig. 4b), are shown as a function of the temperature. The logarithm of the rate was linear (R2 = 0.98). In other words, the rate k increased exponentially with the temperature. The logarithm of the maximum increased with the temperature from 50 to 55°C and reached a maximum for temperatures higher than 55°C. As a consequence, it is independent of the temperature for temperatures higher than 55°C, up to 65°C. In fact, it was observed that for lethal heat treatments, the mean and standard deviation of τa are independent of the decimal reduction or the temperature of the heat treatment.
FIG. 4.
Secondary modeling. The logarithm of the rate k (min−1) (a) and the logarithm of the maxima of τa (h) (b), as defined in Fig. 3, are plotted as a function of the temperature. ln(k) increases linearly with the temperature, so k increases exponentially with the temperature. The maxima are asymptotic: i.e., they do not depend on the temperature for temperatures higher than 55°C.
DISCUSSION
On the choice of the distribution of single-cell lag times.
The repair time that each cell has to undergo after the heat treatment before being able to divide again was mathematically formalized by the choice of the distribution, this being the shifted gamma distribution. The lag of single cells of Lactobacillus after a heat treatment was previously modeled with an extreme value distribution (27). This distribution has the advantage of having an explicit function to characterize the minimum of the lag times but does not show the occurrence of a repair time. The distribution of single-cell lag times of Listeria after stresses of a different nature or a succession of stresses including heat stress was also modeled with the extreme value type II distribution with a fixed shape parameter (14). No shift was included either.
In addition, by assuming a shifted gamma distribution for the single-cell lag times, it is possible to calculate the parameters of the distribution from the detection times measured with the Bioscreen with explicit expressions of the three moments of the distribution without making any assumption on the shape of the distribution (Baranyi et al., submitted).
On the shape of the distribution or CV.
The advantages and disadvantages of the Bioscreen technique and the possible causes of distortion of the single-cell lag times estimated from the detection times have already been discussed extensively elsewhere (18, 19, 25). Previously reported CV for individual cell lag times vary widely from one study to another, with values ranging from 0.09 to greater than 1.0 depending on the stress, growth conditions, and measurement methods (7, 9-11, 14, 15, 20, 27, 28, 31). However, it has been shown that the CV could be assumed to be constant in particular sets of experiments (7, 11, 14, 18). We found here that the shape of the distribution was practically constant, and hence so was the CV. With the assumption of a constant shape parameter, the number of regressed parameters is decreased and the robustness of the model is increased.
On the effect of heat stress on the CV of the distribution of the single-cell lag times.
The CV has been shown to increase after heat stress—from 0.09 to 0.3 after a sublethal heat treatment of Lactobacillus (27)—or to be constant—0.2 after heat treatments of Listeria innocua at 55°C to 62°C in paté and dairy products (7) and after sublethal heat treatment of Escherichia coli in broth (21). However, in these cases, the shift was not taken into account in the modeling, the incubation temperatures were different, and the measurement methods were different, and as the recovery medium has an effect too, it is difficult to compare the results. However, qualitative features were in agreement: e.g., there was no significant difference between the effects of the treatments in dairy products at 62 and 65°C, both leading to a three-decimal reduction in cell numbers, which is consistent with what we found here.
Repair time and stress.
For lethal heat treatments, the standard deviation and the mean of the adjustment period are independent of the heat treatments, so only the repair times vary with the severity of the heat treatment. We observed that the population lag and the repair time increased at the same rate as a function of the decimal reduction in cell numbers only, independently of the time-temperature combination at which they have been obtained. The repair time is an important part of the population lag time as it represents about 75% of the population lag time.
For sublethal heat treatments, we observed that the repair time and the mean and the variance of the single-cell adjustment time increased with the severity of the heat treatments. The repair time increased in the same way as for lethal heat treatment as equation 3 is valid for both lethal and sublethal heat treatments, so there is no discontinuity or change of trend as the heat treatments shift from sublethal to lethal.
Relationship with the F value.
In fact, if we examine equation 3 closely, we can see that the repair time is a function of the F value. The F value was introduced in the canning industry to characterize the lethality achieved by heating for different combinations of time and temperature. It is defined as the equivalent number of minutes heating at a reference temperature (Tref), this usually being 121.1°C
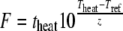 |
(5) |
where T is the temperature and t is the heating time.
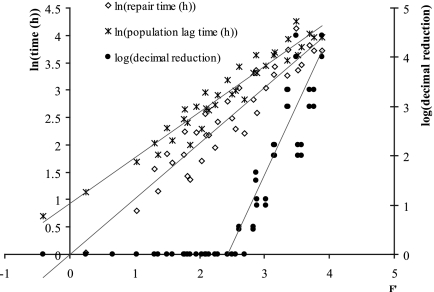
Thus, thermal processes with the same F value achieve the same number of decimal reductions, irrespective of the particular combinations of times and temperatures used in the processes. Figure 5 shows the decimal reduction, the logarithm of the repair time, and the logarithm of the population lag times as a function of F′, (equation 3) with F′ = 1.53 log(theat) + 0.327 Theat − 16.76.
FIG. 5.
The logarithm of the population lag time and the logarithm of repair time are plotted against F′, a reparameterization of the F value which quantifies the cell damage including for sublethal heat treatment, z′ being a generalized z value. Both the repair time and the equivalent population lag time increase linearly with F′.
F′ can be rewritten as
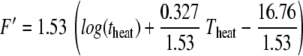 |
that is F′ = 1.53 log(F) with z′ = 4.68°C.
Since the logarithm of the inactivation coefficients is proportional to the temperature, the reference temperature is not important. From the thermal inactivation data, we found that z = 5.73°C (R2 = 0.95). The generalized z value from the secondary model, z′ = 4.68°C, falls within the 95% confidence limits of the z value calculated from the thermal inactivation. The implication of this is that the damage to cells can be characterized by the repair time and the F value concept extended to sublethal heat treatment.
On the modeling of sublethal injury.
Based on the multiple-hit model for sublethal injury introduced by Gould (13), we may interpret our data as follows: cells receive sublethal “hits” at random, and the rate of increase of sublethal hits increases exponentially with temperature as it does with lethal hits. If we further suppose that the repair time is proportional to the number of sublethal hits sustained by a cell, then the cumulative sublethal damage and hence the duration of lag can be expressed in terms of an F value irrespective of heating time or temperature. This is what was observed here.
The population-equivalent lag time increased linearly with F′ but at a slower rate than the repair time, τr. In the simple model presented here, the adjustment time, τa, is treated independently of τr, but in reality, both processes may overlap in a recovering cell such that in cells with long lag times adaptation may be taking place before repair proper is complete.
Consequence for food safety.
For lethal heat treatments, the repair time depends only on the decimal reduction, not the temperature at which the cells were heated. The adjustment period does not depend on the heat treatment at all, so whether the heat treatment is longer at lower temperature or shorter at higher temperature but resulting in the same decimal reduction, the distribution of the lag times from low inocula is the same. Provided that heat-injured cells in food behave in a similar way to those examined here, the distribution of the times to a potentially infectious dose will depend on the severity of the heat treatment but not on the particular times and temperatures used in the thermal treatment. These results are limited to the range of temperature and length of heat treatment studied here: they do not include the conditions under which the population lag times were found to have decreased after the heat treatments (4).
To conclude.
The systematic measurement of the effect of the severity of heat treatment on the variation of lag times within a population presented in this paper allowed us to model the effect of heat treatments and interpret the results according to the random inactivation of sublethal targets within the cell. We have proposed that the lag time can be divided into a repair time and an adjustment period and that the damage caused by the heat treatment can be characterized by the repair time whether the heat treatment is lethal or not. This is an extension of the F value concept that has been used so far for lethal heat treatments only. Although more data are required to validate the model presented, this study used an efficient method and advanced the understanding of the effect of heat treatment on vegetative cells as well as presenting a way of modeling of lag times of single cells after heat treatments.
Acknowledgments
This work was supported by the EU Programme Quality of Life and Management of Living Resources, project no. QLK1-CT-2001-01145 (BACANOVA), project no. FP6-FOOD-023141 (HighQ RTE), and the Biotechnology and Biological Sciences Research Council (grant no. 42266A).
We thank Alexandre Bourrier for invaluable help in carrying out the experiments.
Footnotes
Published ahead of print on 26 September 2008.
REFERENCES
- 1.Baranyi, J., and C. Pin. 2001. A parallel study on bacterial growth and inactivation. J. Theor. Biol. 210:327-336. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 3.Bigelow, W. D., and J. R. Esty. 1920. Thermal death point in relation to time of typical thermophilic anaerobes. J. Infect. Dis. 27:602-617. [Google Scholar]
- 4.Breand, S., G. Fardel, J. P. Flandrois, L. Rosso, and R. Tomassone. 1999. A model describing the relationship between regrowth lag time and mild temperature increase for Listeria monocytogenes. Int. J. Food Microbiol. 46:251-261. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg, R., S. M. George, and M. W. Peck. 1998. Oxygen sensitivity of heated cells of Escherichia coli O157:H7. J. Appl. Microbiol. 85:231-237. [DOI] [PubMed] [Google Scholar]
- 6.Chick, H. 1910. The process of disinfection by chemical agencies and hot water. J. Hyg. (Cambridge) 10:237-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Arrigo, M., G. D. García de Fernando, R. Velasco de Diego, J. A. Ordóñez, S. M. George, and C. Pin. 2006. Indirect measurement of the lag time distribution of single cells of Listeria innocua in food. Appl. Environ. Microbiol. 72:2533-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esty, J. R., and K. F. Meyer. 1922. The heat resistance of the spores of B. botulinus and allied anaerobes. J. Infect. Dis. 31:650-661. [Google Scholar]
- 9.Francois, K., F. Devlieghere, K. Smet, A. R. Standaert, A. H. Geeraerd, J. F. Van Impe, and J. Debevere. 2005. Modelling the individual cell lag phase: effect of temperature and pH on the individual cell lag distribution of Listeria monocytogenes. Int. J. Food Microbiol. 100:41-53. [DOI] [PubMed] [Google Scholar]
- 10.Francois, K., F. Devlieghere, M. Uyttendaele, A. R. Standaert, A. H. Geeraerd, P. Nadal, J. F. Van Impe, and J. Debevere. 2006. Single cell variability of L. monocytogenes grown on liver pate and cooked ham at 7°C: comparing challenge test data to predictive simulations. J. Applied Microbiol. 100:800-812. [DOI] [PubMed] [Google Scholar]
- 11.George, S. M., A. Métris, and S. C. Stringer. 2008. Physiological state of single cells of Listeria innocua in organic acids. Int. J. Food Microbiol. 124:204-210. [DOI] [PubMed] [Google Scholar]
- 12.George, S. M., L. C. C. Richardson, I. E. Pol, and M. W. Peck. 1998. Effect of oxygen concentration and redox potential on recovery of sublethally heat-damaged cells of Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes. J. Appl. Microbiol. 84:903-909. [DOI] [PubMed] [Google Scholar]
- 13.Gould, G. W. 1989. Heat-induced injury and inactivation, p. 11-42. In G. W. Gould (ed.), Mechanisms of action of food preservation procedures. Elsevier Applied Science, London, United Kingdom.
- 14.Guillier, L., and J.-C. Augustin. 2006. Modelling the individual cell lag time distributions of Listeria monocytogenes as a function of the physiological state and growth conditions. Int. J. Food Microbiol. 2006:241-251. [DOI] [PubMed] [Google Scholar]
- 15.Guillier, L., P. Pardon, and J. C. Augustin. 2005. Influence of stress on individual lag time distributions of Listeria monocytogenes. Appl. Environ. Microbiol. 71:2940-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey, B. M. 2000. Injured bacteria, p. 315-341. In B. M. Lund, A. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food, vol. 1. Aspen Publishers, Inc., Gaithersburg, MD. [Google Scholar]
- 17.McKee, S., and G. W. Gould. 1988. A simple mathematical model of the thermal death of microorganisms. Bull. Math. Biol. 50:493-501. [DOI] [PubMed] [Google Scholar]
- 18.Métris, A., S. M. George, and J. Baranyi. 2006. Use of optical density detection times to assess the effect of acetic acid on single-cell kinetics. Appl. Environ. Microbiol. 72:6674-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Métris, A., S. M. George, M. W. Peck, and J. Baranyi. 2003. Distribution of turbidity detection times produced by single cell-generated bacterial populations. J. Microbiol. Methods 55:821-827. [DOI] [PubMed] [Google Scholar]
- 20.Métris, A., Y. Le Marc, A. Elfwing, A. Ballagi, and J. Baranyi. 2005. Modelling the variability of lag times and the first generation times of single cells of E. coli. Int. J. Food Microbiol. 100:13-19. [DOI] [PubMed] [Google Scholar]
- 21.Niven, G. W., J. S. Morton, T. Fuks, and B. M. Mackey. 2008. The influence of environmental stress on the distributions of times to first division in Escherichia coli populations as determined by digital-image analysis of individual cells. Appl. Environ. Microbiol. 74:3757-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peleg, M. 2003. Microbial inactivation curves: interpretation, mathematical modeling, and utilization. Comments Theor. Biol. 8:357-387. [Google Scholar]
- 23.Peleg, M., and M. B. Cole. 1988. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. 38:353-380. [DOI] [PubMed] [Google Scholar]
- 24.Pflug, I. J., and G. W. Gould. 2000. Heat treatment, p. 36-64. In B. M. Lund, A. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food, vol. 1. Aspen Publishers, Gaithersburg, MD. [Google Scholar]
- 25.Pin, C., and J. Baranyi. 2006. Kinetics of single cells: observation and modeling of a stochastic process. Appl. Environ. Microbiol. 72:2163-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahn, O. 1945. Physical methods of sterilization of microorganisms. Bacteriol. Rev. 9:1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smelt, J. P., G. D. Otten, and A. P. Bos. 2002. Modelling the effect of sublethal injury on the distribution of the lag times of individual cells of Lactobacillus plantarum. Int. J. Food Microbiol. 73:207-212. [DOI] [PubMed] [Google Scholar]
- 28.Stephens, P. J., J. A. Joynson, K. W. Davies, R. Holbrook, H. M. Lappin-Scott, and T. J. Humphrey. 1997. The use of an automated growth analyser to measure recovery times of single heat-injured Salmonella cells. J. Appl. Microbiol. 83:445-455. [DOI] [PubMed] [Google Scholar]
- 29.Tomlins, R. I., and Z. J. Ordal. 1976. Thermal injury and inactivation in vegetative bacteria, p. 153-190. In F. A. Skinner and W. B. Hugo (ed.), Inhibition and inactivation of vegetative microbes. Academic Press, London, United Kingdom.
- 30.Whiting, R. C., and R. L. Buchanan. 1994. Microbial modelling. Food Technol. 48:113-120.11539930 [Google Scholar]
- 31.Wu, Y., M. W. Griffiths, and R. C. McKellar. 2000. A comparison of the Bioscreen method and microscopy for the determination of lag times of individual cells of Listeria monocytogenes. Lett. Appl. Microbiol. 30:468-472. [DOI] [PubMed] [Google Scholar]