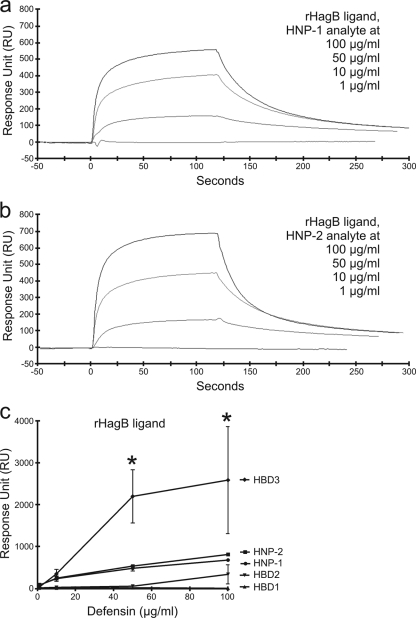
FIG. 1.
SPR spectroscopy sensorgrams showing concentration-dependent binding of HNP-1 and HNP-2 to immobilized rHagB from P. gingivalis. Sensorgrams (a and b) show the overlay of four binding isotherms generated from decreasing concentrations (top line to bottom line, respectively) of HNP-1 and HNP-2. The binding affinity of each defensin for immobilized rHagB was determined by kinetic analysis calculated from the association (Ka) and dissociation (Kd) rates of the four binding isotherms together in each graph using BIAevaluation software version 4.1 (Pharmacia Biosensor AB). HNP-2 and HNP-1 had high affinities characterized by high association and low dissociation rates for immobilized rHagB. The RU signal responses of HNP-2 and HNP-1 in the graph (c) were higher than those previously reported for HBD2 and HBD1 and yet lower than that previously reported for HBD3 (44). *, P < 0.05.