Figure 23.
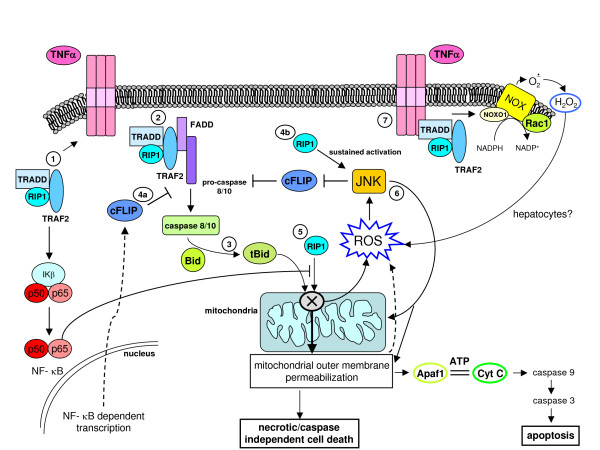
TNF-induced and ROS-dependent events in target cells: the role of JNK activation in mediating cell death. TNF interaction with its type I receptor will generate a sequence of events (depicted in Figure 23) that is not designed to lead uniquely to cell death [197-200] and in which ROS may play a crucial role. 1) The interaction between TNF and TNFRI is first followed by association with the intracytoplasmic receptor tail of the adapter TRADD (TNF-receptor associated death domain), the protein kinase RIP1 and TRAF-2 and TRAF-5 (TNF-receptor associated factors 2 and 5); this works as a signalling complex that activates NF-κB (through Inhibitor of NF-κB kinase (IKK)) and MAPK cascades that regulate the AP-1 transcription factor. Activation of NF-κB-binding activity and transcription of dependent genes is potentially pro-survival by up-regulating the synthesis of cFLIP (c FLICE inhibitory protein), which can inhibit activation of caspase 8/10, the starting point for TNF-mediated induction of classic apoptosis. 2) At this point the TRADD/RIP-1/TRAF-2 complex can dissociates from the receptor by a still unknown mechanism and bind to FADD (Fas-ligand associated death domain) to form another complex leading to recruitment of several molecules of pro-caspase 8/10 and to their autocatalytic cleavage, resulting in activation; however, this event occurs only in cells having low levels of cFLIP, suggesting that apoptosis as well as caspase-independent or necrotic cell death (see later) may occur when the preventive pro-survival function of NF-κB fails. When activation of caspase 8/10 ocurs, the scenario can still prefigure two alternatives. 3) Activated caspase 8/10 may cleave the pro-apoptotic protein Bid (to tBid), which then translocates to mitochondria, causing permeabilization of mitochondrial outer membrane, cytochrome c release, apoptosome assembly and activation of executioner caspases, leading to classic apoptosis. This pro-apoptotic scenario may be amplified by the recruitment to TNFRI of FAN (Factor-associated neutral sphingomyelinase), which is able to produce sphingosine that, in turn, permeabilizes lysosomal membranes, leading to the release of cathepsins. These lysosomal proteases, which can be activated following engagement of different death receptors, act as pro-apoptotic proteases able to cleave Bid as well as to induce oligomerization of Bax or interact directly with mitochondrial outer membrane (note that this pathway, for the sake of clarity, has been omitted from Figure 23). It should be noted that in hepatocytes it has been reported that generation of ROS following activation of death receptors (by Fas or TNF) is strictly dependent on Bid cleavage to tBid and its subsequent translocation to mitochondria [201]. 4) The second alternative is the one more strictly related to ROS and involves the crossroads kinase RIP1. RIP1 has multiple crucial roles; it is involved in the activation of NF-κB, but may also contribute to apoptosis when cleaved by caspase-8 to cRIP1 (4a), an event preventing or reducing activation of NF-κB and sustaining enhanced interaction between TRADD, FADD and procaspases 8/10. However, it has also been described (4b) that the kinase domain of RIP1 is essential to activate JNK, which in turn (although this is just one of the JNK-dependent actions) will favour TNFRI-related apoptosis and mitochondrial outer membrane permeabilization by eliciting cFLIP phosphorylation and degradation. Concerning the involvement of ROS, however, RIP1 (reviewed in [107,193,194]) has been shown (5) to be able also to translocate to mitochondria (prevented by NF-κB) following TNF stimulation of cells: this event induces mitochondrial outer membrane permeabilization and increases release of ROS in the cytoplasm without leading to cytochrome c release and apoptosome complex formation. This increased TNF-related mitochondrial generation of ROS is crucial because it can lead target cells to apoptosis or, more likely, to a necrotic or caspase-independent form of cell death: in this latter scenario a significant role is attributed to ROS-mediated sustained activation of JNK (6), surely operating in hepatocytes [186,187], which, as we will see (Figure 24), is a crucial crossroads for ROS-related irreversible injury of parenchymal cells as elicited by the different aetiologies leading to CLDs. TNF (7) may lead to ROS-dependent and JNK-mediated cell death (necrotic type) also by involving activation of Nox1: however, this mechanism has been described in fibroblasts and, at present, we do not know whether it may apply to hepatocytes or other liver cell populations [195,202].