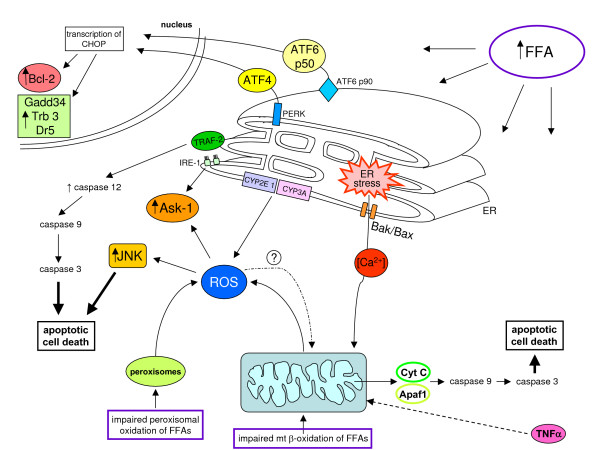
Figure 25.
ER stress and ROS in NAFLD/NASH. This figure describes the most relevant features of ER stress following excess accumulation of FFAs in hepatocytes and also involving oxidative stress and ROS (the latter may originate in NAFLD from deranged mitochondria, CYP2E1 and CYP4A isoforms, or from peroxisomes). On the basis of what is described in the text (sections 'ROS and oxidative stress-related reactive intermediates in NAFLD and NASH: their generation and the role in causing steatosis' and 'Free fatty acids, endoplasmic reticulum stress, oxidative stress and cell death: hepatocyte injury in NAFLD but not only'), the following major features can be offered. a) When the UPR response fails to solve the problem of protein folding caused by different conditions able to induce ER stress (see text for details), including increases in FFA levels, this is followed by an induction of apoptotic cell death that can use both mitochondrial pathways as well as other independent pathways. b) ROS and oxidative stress are able to disrupt ER functions, a major cause seemingly being ROS-dependent increased release of calcium from ER stores: excess calcium has been reported to induce mitochondrial outer membrane permeabilization and, in turn, increased mitochondrial ROS release as a further contribution to increased intracellular levels by other sources and causes operating in NAFLD-related hepatocytes. c) In such a complex scenario, ER stress can result in apoptosis by a number of mechanisms, including: damage to mitochondria leading to cytochrome release, apoptosome formation and related sequential activation of executioner caspase 9 and 3; IRE-1 recruitment of TRAF-2 in order to activate either ASK-1 and then JNK, a potentially pro-apoptotic pathway that can be further sustained by ROS, or (at least in mice) caspase 12, which, in turn, can activate caspases 9 and 3; and activation of PERK and ATF6 (p90), which can lead through nuclear translocation of ATF4 and ATF6(p50), respectively, to transcriptional up-regulation of CHOP, a factor that promotes apoptosis by either inhibiting expression of Bcl-XL or up-regulating expression of pro-apoptotic proteins such as Gadd34, Trb3 and Dr5.