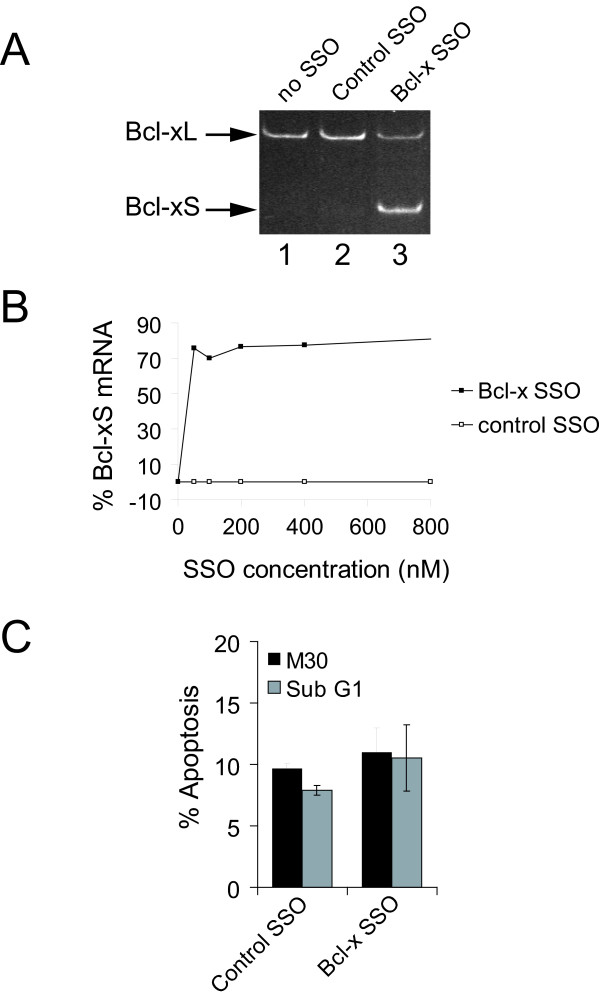
Figure 2.
Specific control of endogenous Bcl-xS splicing by modified antisense RNA oligonucleotides in living cells. (A) Antisense RNA oligonucleotide induces endogenous Bcl-xS mRNA expression. HeLa cells were transfected with 200 nM oligonucleotide directed against the major Bcl-xL splice site (Bcl-x SSO) or a scrambled control oligonucleotide (Control SSO). 24 hours post-transfection total RNA was isolated and subjected to RT-PCR with primers that amplify both the Bcl-xL and the alternative Bcl-xS mRNAs. (B) Concentration-dependent antisense mediated induction of Bcl-xS mRNA expression. HeLa cells were transfected with antisense RNA oligonucleotides as in A. RT-PCR was performed as in A. PCR products were separated by microfluidity and quantified using a 2100 Agilent bioanalyzer. The ratio of Bcl-xS mRNA over total Bcl-x mRNA is expressed on the y-axis. The values from cells treated with scrambled control SSO (open squares), or Bcl-X SSO (black squares) are shown. (C) The effect of endogenous Bcl-xS on apoptosis in HeLa cells. HeLa cells were transfected as in A. Cells were harvested 24 hours post-transfection and percentage of apoptotic cells was analyzed by flow cytometry using monoclonal antibodies that detect caspase cleaved cytokeratin-18 (M30; black bars), or by measuring Sub G1 DNA content (grey bars). Error bars represent the standard deviation of three independent transfections.