Abstract
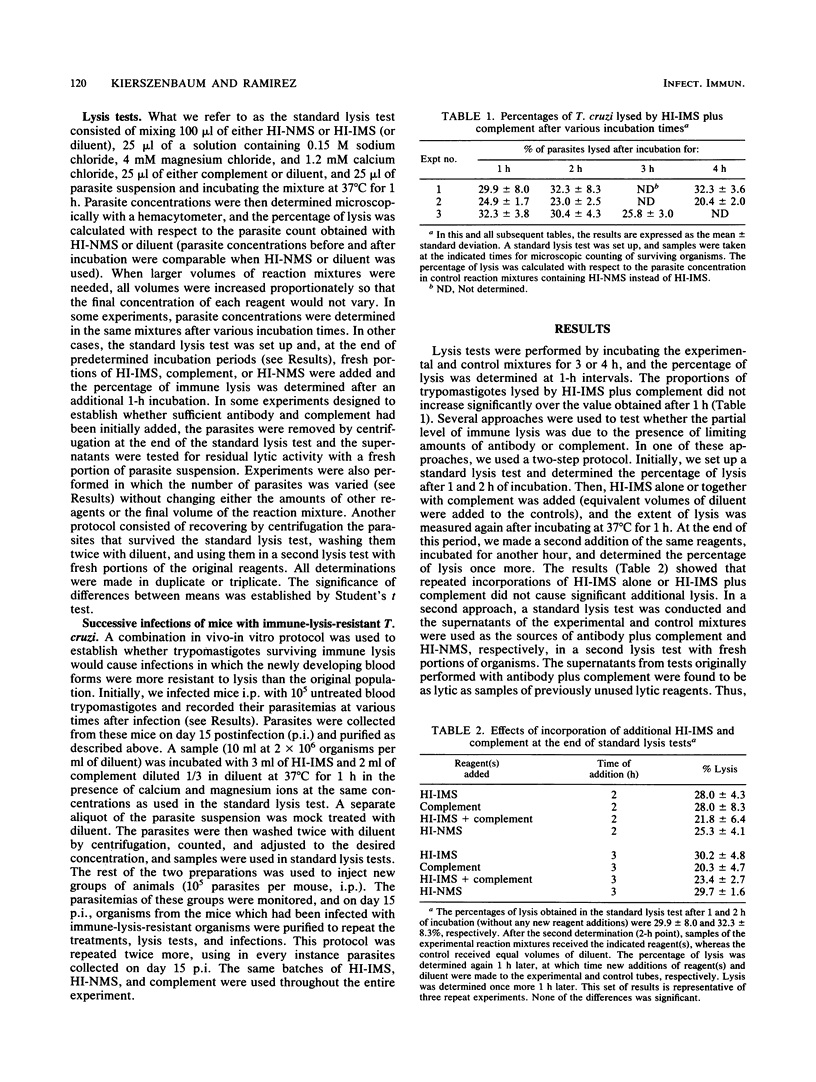
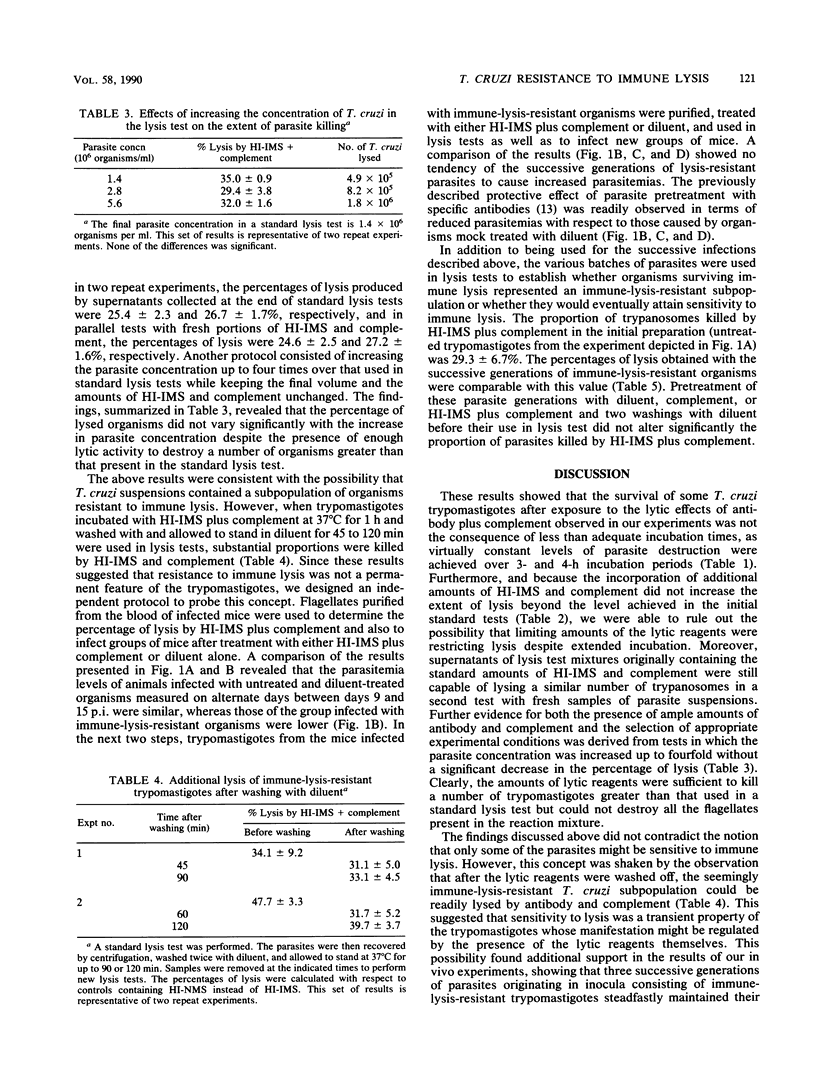
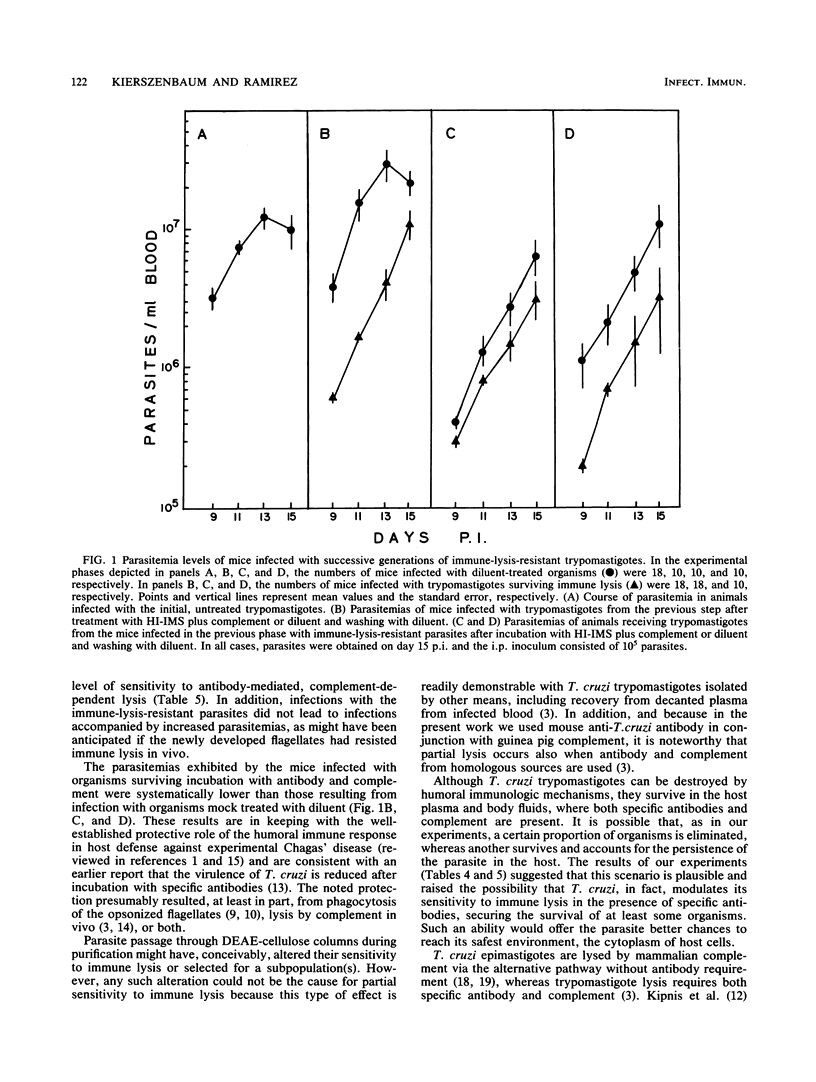
The numerous reports on lysis of blood (trypomastigote) forms of Trypanosoma cruzi by specific antibodies plus complement have systematically shown that a certain proportion of parasites survives. However, it is not known whether the insensitive organisms represent a subpopulation (or clones) or a certain developmental phase of otherwise morphologically identical parasites. In this work, we established that partial lysis was not due to the use of insufficient amounts of lytic reagents. Thus, supernatants of lytic reaction mixtures killed the same proportion of T. cruzi as previously unused reagents. Moreover, in parallel tests in which the trypomastigote concentration was up to four times greater than that used in standard lysis tests, the percentages of lysis were comparable. Incubation periods as long as 4 h did not increase the extent of lysis beyond the value observed after only 1 h, indicating that the routinely used 1-h incubation was appropriate. The extent of lysis was not increased by additional amounts of antibody, complement, or both. Instead, trypomastigotes surviving immune lysis, washed, and incubated with fresh diluent for 45 to 120 min before being used in new lysis tests did manifest additional sensitivity to immune lysis. Three successive infections in mice with parasites which had survived immune lysis led to the production of trypanosomes that displayed the same level of resistance to immune lysis as the original, untreated parasite population. Of interest, the average parasitemias of these groups of mice did not evidence a tendency to increase, as might have occurred if an immune-lysis-resistant subpopulation had been selected. Since trypomastigotes exhibiting resistance to immune lysis can eventually become sensitive, resistance to immune lysis does not represent an insensitive parasite subpopulation. This resistance appears to be modulated by the presence of the lytic reagents and might involve expression of as yet unidentified surface components playing a role in complement activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brener Z. Immunity to Trypanosoma cruzi. Adv Parasitol. 1980;18:247–292. doi: 10.1016/s0065-308x(08)60401-7. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoni R. L., Rottenberg M. E., Segura E. L. A radiometric assay for diagnosing lytic antibodies in Trypanosoma cruzi infection. Parasitol Res. 1988;74(6):512–515. doi: 10.1007/BF00531627. [DOI] [PubMed] [Google Scholar]
- Chao D., Hsu Y. P., Chan C. H., Chen Y. A. Comparative studies on epimastigote and metacyclic stages of Trypanosoma cruzi. II. Selective lysis by rodent sera. Int J Zoonoses. 1985 Dec;12(4):323–330. [PubMed] [Google Scholar]
- Engel J. C., Dvorak J. A., Segura E. L., Crane M. S. Trypanosoma cruzi: biological characterization of 19 clones derived from two chronic chagasic patients. I. Growth kinetics in liquid medium. J Protozool. 1982 Nov;29(4):555–560. doi: 10.1111/j.1550-7408.1982.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Kierszenbaum F. Cross-reactivity of lytic antibodies against blood forms of Trypanosoma cruzi. J Parasitol. 1976 Feb;62(1):134–135. [PubMed] [Google Scholar]
- Kierszenbaum F., Lima M. F. Susceptibility of insect-borne, metacyclic forms of Trypanosoma cruzi to antibody-mediated mechanisms of destruction. Am J Trop Med Hyg. 1983 Nov;32(6):1236–1241. doi: 10.4269/ajtmh.1983.32.1236. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Lima M. F., Wirth J. J. Effects of antiserum to Trypanosoma cruzi on the uptake and rate of killing of vector-borne, metacyclic forms of the parasite by macrophages. Int J Parasitol. 1985 Aug;15(4):409–413. doi: 10.1016/0020-7519(85)90026-8. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Saavedra L. E. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. J Protozool. 1972 Nov;19(4):655–657. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., Krettli A. U., Dias da Silva W. Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand J Immunol. 1985 Aug;22(2):217–226. doi: 10.1111/j.1365-3083.1985.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976 Mar;116(3):755–760. [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- Mercado T. I., Katusha K. Isolation of Trypanosoma cruzi from the blood of infected mice by column chromatography. Prep Biochem. 1979;9(1):97–106. doi: 10.1080/00327487908061675. [DOI] [PubMed] [Google Scholar]
- Murfin D. J., Kuhn R. E. Relative resistance of Brazil strain trypomastigote forms of Trypanosoma cruzi to in vitro antibody-dependent complement-mediated lysis. J Parasitol. 1988 Dec;74(6):1046–1050. [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Güther M. L., Yoshida N. Mechanism of resistance to lysis by the alternative complement pathway in Trypanosoma cruzi trypomastigotes: effect of specific monoclonal antibody. J Immunol. 1986 Sep 1;137(5):1623–1628. [PubMed] [Google Scholar]
- Yoshida N. Trypanosoma cruzi: recognition of trypomastigote surface antigens by lytic antisera from mice resistant to acute infection. Exp Parasitol. 1986 Apr;61(2):184–191. doi: 10.1016/0014-4894(86)90151-7. [DOI] [PubMed] [Google Scholar]


