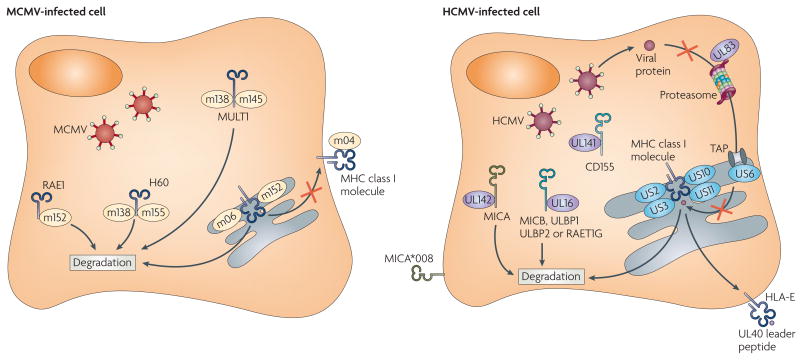
Figure 2. HCMV and MCMV proteins affecting NK-cell-mediated recognition of virus-infected cells.
The virus-encoded proteins m04, m06 and m152 inhibit MHC class I expression on the surface of mouse cytomegalovirus (MCMV)-infected cells by a complex process that differs depending on the MHC class I alleles present in the host22. m04 can be expressed on the cell surface of MCMV-infected cells in a complex with MHC class I molecules; however, how this influences the recognition of MHC class I molecules by receptors on natural killer (NK) cells or T cells is unknown. Human cytomegalovirus (HCMV) also blocks the expression of MHC class I molecules in infected cells in an allele-specific manner (reviewed in REF. 65). The HCMV proteins US2, US3, US10 and US11 interact with the MHC class I heavy chains on their own or with the heavy chains complexed with β2-microglobulin, ultimately resulting in their degradation, whereas US6 blocks TAP (transporter associated with antigen processing) function and UL83 inhibits protein entry into the proteasome. UL40 provides a leader peptide that binds to HLA-E allowing its expression on the surface of HCMV-infected cells, presumably for interactions with the inhibitory CD94-NKG2A(NK group 2, member A) receptor on NK cells. Both MCMV and HCMV inhibit expression of the NKG2D ligands in infected cells. The MCMV-encoded m152 protein targets RAE1 (retinoicacid early transcript 1), as well as MHC class I molecules, for degradation; m145 and m138 cooperate to prevent MULTl (murine UL16-binding protein (ULBP)-like transcript 1) expression; and m138 and m155 cause degradation of H60. In humans, the HCMV-encoded UL16 protein inhibits expression of MICB (MHC-class-I-polypeptide-related sequence B), ULBP1, ULBP2 and RAET1G (retinoic-acid early transcript 1C), whereas UL142 prevents expression of MICA. Certain allelesof MICA, such as the common allele MICA*008, are resistant to downregulation by HMCV because of a truncation of the cytoplasmic domain. CD155, a ligand for the activating NK-cell receptors DNAM1 (DNAX adhesion molecule 1)and CD96, is targeted by UL141.