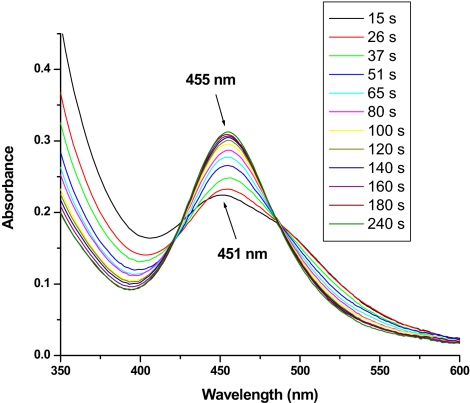
Fig. 3.
Spectral changes after the addition of 30 eq of Ce(IV) to a solution containing 1.25 × 10−5 M (H2O)RuIIIORuIII(OH2)4+ and 1.25 × 10−5 M [Ru(bpy)2(bpz)]2+ as redox mediator in 1.0 M HNO3. Initially, the peroxidic intermediate (HO2)RuIVORuIV(OH)4+ (λmax = 451 nm) is the dominant species in solution, because its oxidation by the redox mediator is the rate-limiting step. As a result of a higher anion concentration in this case (1.0 M NO3−), the anated species (O2NO)RuIVORuIV(OH)4+ (λmax = 455 nm) begins to form before all of the Ce(IV) has been consumed (≈180 s), and it is the dominant form of the dimer at the end of the experiment (≈300 s).