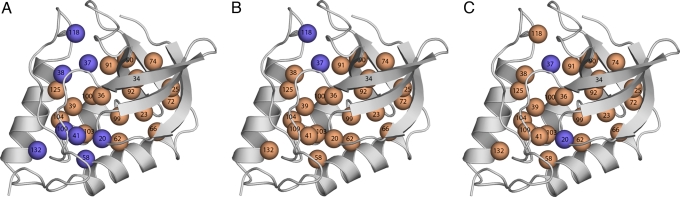
Fig. 3.
pH dependence of difference thermodynamic stability [ΔΔG°H2O = ΔG°H2O (variant) − ΔG°H2O (background)], defined as (ΔΔΔG°H2O/ΔpH) = (ΔΔG°H2O, pH1 − ΔΔG°H2O, pH2)/(∣pH1 − pH2∣). pH1 = 7 and pH2 = 5 for Asp and Glu or 10 for Lys. Blue circles identify positions where ΔΔΔG°H2O/ΔpH = 0, indicating that the pKa values are probably normal; orange circles identify positions where ΔΔΔG°H2O/ΔpH > 0.2, indicating that the pKa values are shifted in the direction that favors the neutral state (depressed for basic groups, elevated for acidic ones). Data are for variants with internal Lys (A), Glu (B), and Asp (C).