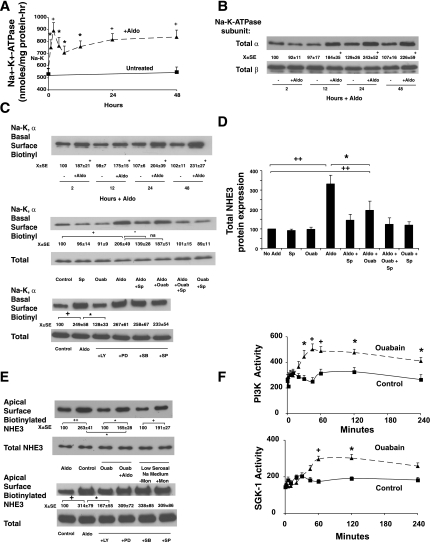
Fig. 3.
Aldosterone stimulates Na+-K+-ATPase activity by stimulating exocytosis of the α-subunit, contributing to NHE3 induction. A: cells were treated with 1 nM aldosterone, cells were harvested at varying times, and K+-stimulated phosphatase activity, a measure of Na+-K+-ATPase activity, was measured in postmitochondrial membranes as described in materials and methods. Values shown are means ± SE of 3 separate experiments. *P < 0.05 and +P < 0.01 compared with activity at zero time by analysis of variance. B: aldosterone stimulates a time-dependent increase in the α-subunit, but not β-subunit, of Na+-K+-ATPase protein expression. Western blots shown are representative of 3 experiments. Densitometry values shown are means ± SE. C: aldosterone stimulates basolateral membrane (BLM) insertion of the α-subunit of the Na+-K+-ATPase as early as 2 h, which continues up to 48 h (top, Western blot). Samples at 2 h were analyzed further (middle, Western blot) to determine the basis of increased Na+ pump activity (see A). Surface expression of the α-subunit was assessed through BLM biotinylation and total expression of α-subunit and β-subunit measured in lysates. Cells were stimulated with 1 nM aldosterone for 2–48 h, and surface expressed proteins on the basolateral side were labeled with Sulfo-normal horse serum (NHS)-biotin as described in materials and methods; total cellular expression was determined in total cell lysates. To determine effects of transduction kinase inhibitors, cells were pretreated with the PI3K kinase inhibitor (L294002, 30 μM) or MEK-1 inhibitor (PD98059, 30 μM), the p38 MAP kinase inhibitor (SB203580, 30 μM), or the SAPK/JNK MAP kinase inhibitor (SP600125, 30 μM) for 2 h before aldosterone treatment (1 nM), and basolateral surface biotinylation was performed after 2-h aldosterone stimulation. +P < 0.01 compared with untreated at same time point by analysis of variance for B and C, top (time course). D: spironolactone and ouabain inhibited aldosterone-induced increases in total cellular NHE3 expression at 24 h. Cells were treated with ouabain and/or spironolactone alone or before aldosterone, and total cell NHE3 expression was determined 24 h later. E: aldosterone-stimulated apical NHE3 insertion is inhibited by ouabain and could be mimicked by low Na+ basolateral medium and the Na+/H+ ionophore monensin. Monolayers were stimulated with aldosterone as previously; in those cases noted monolayers were treated with ouabain (100 μM) for 2 h before 2-h aldosterone stimulation or treated only with ouabain for 4 h. For monolayers and low serosal Na+ medium, media were changed to HCO3− medium with 140 mM Na+ apically and 5 mM Na+ basolaterally for 2 h, and, when appropriate, monensin (3 μM) was added to the basolateral medium (or ethanol vehicle). To determine effects of transduction kinase inhibitors, cells were pretreated with the PI3K kinase inhibitor (LY294002, 30 μM), MEK-1 inhibitor (PD98059, 30 μM), the p38 MAP kinase inhibitor (SB203580, 30 μM), or the SAPK/JNK MAP kinase inhibitor (SP600125, 30 μM) for 2 h before aldosterone treatment (1 nM), and apical surface biotinylations were performed after 2 h of stimulation. F: cells were untreated (control) or stimulated with ouabain (100 μM) and harvested at varying times, and PI3K and SGK-1 activities were measured as described in materials and methods. Images shown are representative of 5 separate experiments and are means ± SE. *P < 0.05, + P < 0.01, and ++P < 0.001 statistical comparisons by analysis of variance with a Bonferroni correction with groups designed by bars in C, bottom (2-h inhibitor data) and D and E. For F, comparisons were made by paired Student's t-test.