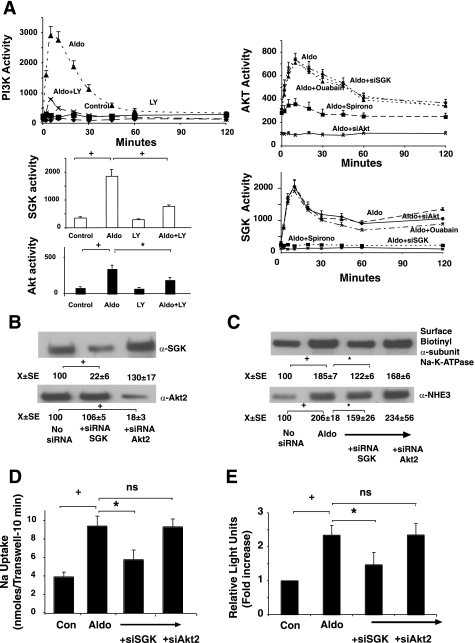
Fig. 4.
Aldosterone stimulates PI3K, serum glucorticoid kinase (SGK), and Akt-2 activities, but only SGK via Na+-K+-ATPase stimulation contributes to induction of NHE3. A: cells were stimulated with 1 nM aldosterone, and cell lysates were harvested at varying times; PI3K, SGK, and Akt-2 kinase activities were measured at varying times by immunoprecipitating the kinases and using a peptide substrate. When appropriate, cells were treated with the PI3K inhibitor LY294002 (30 μM for 2 h), ouabain (100 μM for 2 h), or spironolactone (10 μM for 2 h) or had SGK-1 or Akt-2 kinases silenced (siRNA). Values are activity to transfer [32P] from ATP to the phosphatidyl inositol acceptor (PI3K assay) or synthetic peptide substrates and are means ± SE of 3 separate experiments. B–D: silencing SGK but not Akt-2 activity inhibits aldosterone-stimulated insertion of Na+-K+-ATPase α-subunit into the basolateral membrane. SGK-1 or Akt-2 expression were silenced with silencing oligonucleotides (confirmed by Western blots presented in B and activity silenced presented in A), and, 24 h after second oligo introduction, cells were stimulated by 1 nM aldosterone and basolateral surface Na+-K+-ATPase α-subunit expression determined 2 h later as described in materials and methods (C), total cell NHE3 expression (C), and activity (D) as well as NHE3 promoter activity determined by luciferase transfections (E) measured 24 h later. Images shown are representative of 3 separate experiments. Flux or luciferase values are means ± SE for 3 separate experiments. *P < 0.05, +P < 0.01, and ++P < 0.001 for comparisons as indicated by bars between groups by analysis of variance with a Bonferroni correction for B–E.