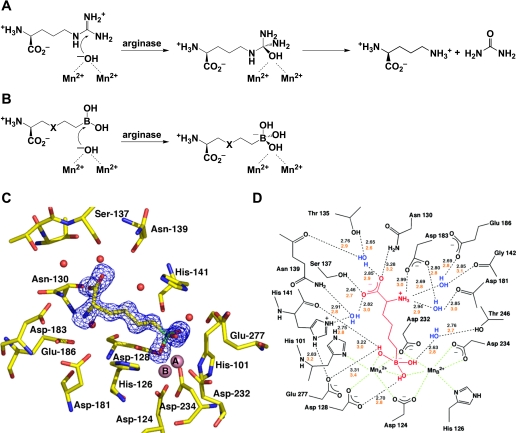
Fig. 2.
A: arginase catalyzes the nucleophilic attack of a metal-bridging hydroxide ion at the guanidinium group of l-arginine to yield l-ornithine and urea. B: boronic acid analogues of l-arginine, 2(S)-amino-6-hexanoic acid (ABH) (X = CH2) and S-(2-boronoethyl)-l-cysteine (BEC) (X = S), undergo nucleophilic attack by the metal-bridging hydroxide ion in the arginase active site to yield tight-binding boronate anions. C: electron density map of ABH bound to human arginase I, confirming the binding of the tetrahedral boronate anion. [From Di Costanzo et al. (26).] D: scheme showing enzyme-inhibitor hydrogen bond (black dashed lines) and metal coordination interactions (green dashed lines) observed in arginase I-ABH complexes. [From Cox et al. (19) and Di Costanzo et al. (26).] On average, shorter enzyme-inhibitor hydrogen bond interactions are observed in the human arginase I-ABH complex (black numbers) compared with the rat arginase I-ABH complex (orange numbers). This structural feature may account for the higher affinity of ABH toward human arginase I (Kd = 5 nM) compared with rat arginase I (Kd = 110 nM).