Abstract
This is the first description of functional immunoassay technology (FIT), which as a diagnostic tool has broad application across the whole spectrum of physiological measurements. In this paper, FIT is used to measure the renal clearance of an ultra low-dose administration of a clinically available contrast reagent for the purpose of obtaining an accurate glomerular filtration rate (GFR) measurement. Biomarker-based GFR estimates offer convenience, but are not accurate and are often misleading. FIT overcomes previous analytic barriers associated with obtaining an accurate GFR measurement. We present the performance characteristics of this diagnostic test and demonstrate the method by directly comparing GFR values obtained by FIT to those obtained by an FDA approved nuclear test in 20 adults. Two subjects were healthy volunteers and the remaining 18 subjects had diagnosed chronic kidney disease, with 12 being kidney transplant recipients. Measured GFR values were calculated by the classic UV/P method and by the blood clearance method. GFR obtained by FIT and the nuclear test correlated closely over a wide range of GFR values (10.9–102.1 ml·min−1·1.73 m−2). The study demonstrates that FIT-GFR provides an accurate and reproducible measurement. This nonradioactive, immunoassay-based approach offers many advantages, chiefly that most laboratories already have the equipment and trained personnel necessary to run an ELISA, and therefore this important diagnostic measurement can more readily be obtained. The FIT-GFR test can be used throughout the pharmaceutical development pipeline: preclinical and clinical trials.
Keywords: renal function, chronic kidney disease, transplant
the measurement of physiological systems is important in biomedical research and has application in clinical diagnostics. However, the ability to measure many kinetic processes in vivo has been restricted to laboratories having either the ability to use radioactive materials or the capability of maintaining expensive and specialized analytic equipment. In this report, we present a new approach to measure physiological systems that offers a greater level of access. This approach is called functional immunoassay technology (FIT) that combines three simple concepts which together become a powerful tool to facilitate many physiological tests (9). The first step of FIT requires an administration of a nonradioactive xenobiotic reagent designed to measure a specific physiological parameter. The second step of FIT uses immunoassay as the readout system to quantify the concentration of the xenobiotic reagent in samples-of-interest collected at specific time intervals post administration. The third step uses these readouts as inputs to calculate the functional parameter for the system-of-interest. Examples of functional measurements wherein FIT has application include, but are not limited to, the measurement of glomerular filtration rate, renal blood flow, intestinal transit, plasma volume, total fluid volume, membrane permeability, and hepatic receptor activity. FIT provides a nonradioactive platform that can be readily adopted by most research and clinical laboratories. To demonstrate the concept of FIT, we applied this technology for the measurement of glomerular filtration rate (GFR).
An accurate measurement of GFR is critical for the management of patients with chronic kidney disease (CKD), renal transplant recipients, and the evaluation of potential transplant donors. This diagnostic test is also needed in renal physiology research and in clinical trials of pharmaceutical agents, where accurate assessment of renal function is important. Estimated GFR derived by evaluating the fluctuation of naturally occurring biomarkers have been shown to provide limited diagnostic value for these applications (8, 11–17, 20), as highlighted in a recent systematic review of 23 studies concerning the performance of creatinine-based GFR estimates in kidney transplant recipients (21).
After careful review by the National Kidney Foundation, the direct measurement of GFR remains the “gold standard” for assessing kidney function (14). The diagnostic procedure to accurately measure GFR is simple and straightforward. The procedure requires the administration of a GFR probe followed by timed blood or blood and urine collections. The historical barrier to universal use of a measured GFR test has been its association with radioactivity or the unsuitability of the analytic component of a nonradioactive test precluding its adoption into a routine clinical laboratory due to the requirement for specialized equipment, complex analytical procedures, and slow sample throughput.
In this report, we describe and present the performance characteristics of the FIT-GFR test. Because this diagnostic test has applications in basic research, veterinary medicine and human clinical research, we also evaluate potential interference from different species serum. As part of our initial validation, we present the results of a clinical study that demonstrate the accuracy of the FIT method to measure GFR in humans. The study comprised healthy volunteers and patients with diagnosed CKD many of whom are kidney transplant recipients. The results of this study show that the FIT-GFR method provides an accurate measurement over a wide range of GFR values.
MATERIALS AND METHODS
FIT-GFR Kit Components
ELISA FIT-GFR kit.
The FIT-GFR kit (BioPAL, Worcester, MA) contains gadopentetate dimeglumine (Gd-DTPA) concentrate, rabbit anti-Gd-DTPA antiserum, HRP-Gd-DTPA conjugate, and a goat anti-rabbit IgG-coated 96-well plate. The composition of each component is described in the package insert of the kit.
FIT-GFR Kit Validation
Sample interference.
Sample interference was evaluated for rat, mouse, feline, canine, and human serum. Samples were diluted 1:0, 1:10, 1:20, 1:50, 1:100 into sample diluent. Fifty microliters of diluted sera sample were pipetted into sample wells in triplicate and processed following the standard assay protocol. The resulting optical density values were compared with the mean zero standard value.
Cross-reactivity.
The cross-reactivity of a number of chelates with anti-Gd-DTPA antiserum were assessed. Among the compounds tested were gadolinium, DTPA, Gd-DTPA, DOTA, Gd-DOTA, EDTA, Gd-EDTA, citrate, and succinate. Various common drugs, such as aspirin, Tylenol, and ibuprofen were also tested.
Sensitivity.
As previously described by Anderson (2), the limit of detection (LOD) and the limit of quantitation (LOQ) were determined by measuring 15 blank replicates of human serum containing no Gd-DTPA at two dilutions (undiluted and 1:300), 15 blank replicates of human urine containing no Gd-DTPA at two dilutions (undiluted and 1:3,000), and 15 blank diluent samples. The standard curve was run in triplicate. The mean value and standard deviation (SD) for each sample set were determined. The LOD was calculated as the means ± 3SD, and the LOQ calculated as the means value ± 10SD.
Accuracy and precision.
Intra-assay replicate analysis (n = 10) and interassay replicate analysis (n = 5) at two levels of Gd-DTPA (low 0.007 μg/ml; high 0.065 μg/ml) were determined. The interassay replicates were collected over 5 mo. Accuracy was defined as the range of percentage differences between the means ± 2SD of back-calculated concentrations and real standard values. Intra-assay and interassay precision were expressed as the percent coefficient of variation (CV) of the measured Gd-DTPA concentration, i.e., %CV = 100·SD/mean.
Comparison Study
Experimental protocol.
The study was approved by the Internal Review Board and was performed in adherence to the Declaration of Helsinki, and informed consent was obtained from all subjects. The investigation was performed using 20 subjects (14 men), ranging from 30–71 yr of age. Two subjects were healthy volunteers with no known history of renal disease or diabetes. The remaining 18 subjects had diagnosed CKD, wherein 8 were diabetic, 12 received a kidney transplant, and 1 received a liver transplant. All subjects received Lugol's solution before the start of the study to block thyroidal uptake of radioactive 125I. The height and weight of each subject were measured, and subjects were encouraged to drink water throughout the study.
[125I]iodothalamate (10 μCi, Glofil, IsoTex Diagnostics) and gadopentetate dimeglumine (10 μl/kg, 5 μmol/kg, Gd-DTPA, Magnevist, Berlex) were intravenously administrated to each subject. After injection, blood and urine samples were collected, as shown in Fig. 1. The dose of Gd-DTPA used in the study is 10× lower than the recommended dose for magnetic resonance imaging (MRI). The exact time of the injection and each blood and urine collection was recorded, and the volume of each urine collection was measured. Blood was collected in serum separation tubes without anticoagulant and centrifuged within 1 h.
Fig. 1.
Timeline of the study.
Radiation counting for [125I]iodothalamate.
One milliliter of serum and 1 milliliter of urine from each collection were prepared for radiation counting (Cobra II Auto-Gamma, Packard Instruments). All samples were counted twice for 5 min and corrected for tracer decay during the counting period.
ELISA procedure for FIT-GFR.
Standards were prepared using BioPAL's diluent (0.003, 0.01 0.03, 0.1, 0.3 μg/ml). Using the same diluent, serum and urine samples were diluted. It was determined that serum required a dilution of 1:300 and urine samples required a dilution of 1:3,000 to bring the samples within the active range of the standard curve. Fifty microliters of the standard or diluted sample were combined in sequence in goat anti-rabbit IgG-coated plate wells with 50 μl of HRP-Gd-DTPA conjugate containing a yellow dye and 50 μl of rabbit anti-Gd-DTPA containing a blue dye. Upon combination, the wells containing all reagents became green and were incubated for 90 min at 25°C. The wells were washed with a Tween 20 PBS buffer (Elx50, Biotek Instruments, Winooski, VT). Substrate (100 μl) was added to all wells and incubated for 30 min. Stop reagent (100 μl) was then added, and the optical density at 450 nm for each well was recorded. Using software supplied by the plate reader (Multiskan Spectrum, Thermo Election), data from the standards were fit to a four-parameter logistic function. By interpolation, the concentration of Gd-DTPA present in each sample was determined. The analytic procedure required ∼3 h to compete.
GFR calculation via UV/P method.
The protocol used in this study provides three UV/P measurements, as follows:
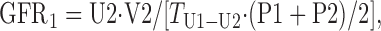 |
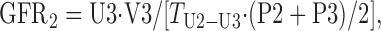 |
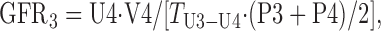 |
where U and P are the concentration of either [125I]iodothalamate (cpm/ml) or Gd-DTPA (μg/ml) in urine or serum, respectively; V is the volume (ml) of urine collected at each time point; and T is the time duration between urine collections (min). The three GFRs (ml/min) were averaged and then normalized per 1.73 m2 of body surface area (BSA), i.e., (ml/min)·1.73/BSA = ml·min−1·1.73 m−2.
GFR calculation via blood clearance method.
A one-compartment blood clearance method was used in this study (3). The concentration of [125I]iodothalamate (cpm/ml) and Gd-DTPA (μg/ml) in each blood sample was plotted as a function of time. The data were fit to a one-exponential decay function, i.e., Y = Be−bX. The function was integrated over the limits zero to infinity to obtain the area under the curve (AUC), i.e., AUC = B/b (mg·min−1·ml−1). The GFR value is then obtained by dividing the administrated dose by the AUC. The GFR (ml/min) is then normalized per 1.73 m2 of BSA.
Sample stability.
Blood and urine samples from selected patients were stored at 22, 4, and −20°C. After 1 mo, samples were removed from storage and the FIT analysis was repeated and compared with the original value.
Data analysis.
GFR values obtained by [125I]iodothalamate were considered the clinical standard and were directly compared with GFR values obtained by FIT using the analysis of Bland and Altman (4). FIT-GFR values were compared with the clinical standard by assessing the bias of the GFR test (the difference between the GFR test and the clinical standard) and the precision between the GFR test and the clinical standard (r2), with the accuracy expressed as the percentage of the GFRs that fell within 20% of the clinical standard. In addition, Student's t-test was also used to evaluate statistical differences between methods.
RESULTS
FIT-GFR Kit Performance
The corresponding LOD for diluent and human serum (undiluted and 1:300) and urine (undiluted and 1:3,000) was ∼0.0010 μg/ml for each matrix. The LOQ for diluent was 0.0024 μg/ml, for human serum (undiluted and 1:300) were 0.0031 and 0.0020 μg/ml, respectively, and for urine (undiluted and 1:3,000) were 0.0036 and 0.0024 μg/ml, respectively. The presence of human serum or urine did not have a significant effect on the baseline measurement. Moreover, for additional species tested, there was no measurable sample interference observed with serum at any dilution level, including undiluted serum.
The optical density readout and the corresponding Gd-DTPA concentration for the replicates from the intra-assay analysis of the low and high controls are provided in Table 1. The intra-assay %CVs for the low and high controls were 4.3 and 2.3, respectively, and the corresponding measured Gd-DTPA concentrations were 0.0076 ± 0.0003 and 0.0660 ± 0.0015 μg/ml, respectively. For the interassay analysis, the measured Gd-DTPA concentrations for low and high controls were 0.0075 ± 0.0005 and 0.0627 ± 0.0043 μg/ml, respectively, corresponding to interassay %CVs of 6.7 and 6.8, respectively.
Table 1.
Optical density and concentration for Gd-DTPA from replicates from intra-assay analysis of low and high controls
|
Low Control |
High Control
|
|||
|---|---|---|---|---|
| Optical Density | Concentration, μg/ml | Optical Density | Concentration, μg/ml | |
| 1 | 0.6841 | 0.0075 | 0.3132 | 0.0648 |
| 2 | 0.6720 | 0.0082 | 0.3035 | 0.0686 |
| 3 | 0.6812 | 0.0077 | 0.3071 | 0.0671 |
| 4 | 0.6833 | 0.0075 | 0.3069 | 0.0672 |
| 5 | 0.6771 | 0.0079 | 0.3089 | 0.0664 |
| 6 | 0.6935 | 0.0070 | 0.3099 | 0.0660 |
| 7 | 0.6864 | 0.0074 | 0.3125 | 0.0650 |
| 8 | 0.6812 | 0.0077 | 0.3169 | 0.0634 |
| 9 | 0.6765 | 0.0079 | 0.3092 | 0.0663 |
| 10 | 0.6824 | 0.0076 | 0.3126 | 0.0650 |
| Mean | 0.6818 | 0.0076 | 0.3101 | 0.0660 |
| SD | 0.0059 | 0.0003 | 0.0038 | 0.0015 |
| %CV | 0.8 | 4.3 | 1.2 | 2.3 |
CV, coefficient of variation; Gd-DPTA, gadopentetate dimeglumine.
As expected for the cross-reactivity evaluation, Gd-DTPA interacted with the antiserum (100%), as did DTPA (50%). However, the other chelates and commonly used drugs tested did not interact.
Comparison Study
For the 20 human subjects tested, the range of GFR values was 10.9–102.1 ml·min−1·1.73 m−2. The FIT-GFR test provides comparable results to those obtained by the FDA-approved test, demonstrating a high degree of precision and accuracy (Figs. 2 and 3). The comparison of the classic UV/P method shows that the bias between the two tests was −2.07 ml·min−1·1.73 m−2, precision was 3.00 ml·min−1·1.73 m−2, and the accuracy was such that 95% of the subjects had FIT-GFRs within 20% of the nuclear test (see Fig. 2B). For the blood clearance method, the bias between the two tests was −4.58 ml·min−1·1.73 m−2, precision was 6.16 ml·min−1·1.73 m−2, and the accuracy was such that 90% of the subjects had FIT-GFRs within 20% of the nuclear blood clearance test (see Fig. 3B). There was no statistical difference measured between FIT-GFRs obtained by either UV/P or the blood clearance method compared with the clinical standard.
Fig. 2.
Functional immunoassay technology (FIT) and [125I]iodothalimate glomerular filtration rate (GFR) values (ml·min−1·1.73 m−2) calculated by the UV/P method. A: FIT-GFR values are directly compared with [125I]iodothalimate GFR values y = 1.85 + 1.00x; r2 = 0.99; P = 0.81. B: difference against the mean for FIT-GFR values.
Fig. 3.
FIT and [125I]iodothalimate GFR values (ml·min−1·1.73 m−2) calculated by the blood clearance method. A: FIT-GFR values are directly compared with [125I]iodothalimate GFR values y = −4.02 + 1.09x; r2 = 0.95; P = 0.62. B: difference against the mean for FIT-GFR values.
Sample Stability During Storage
After 1 mo, there was no significant change between the first and second FIT-GFR values observed with samples stored at 22 and 4°C. However, samples stored at −20°C proved to be unstable.
Serum and urine samples that were stored at 4°C were diluted 1:10 with a 0.01 M Tris buffer (pH 7.4) containing 10% glycerol and then stored at −20°C. After 1 mo, there was no significant change between the first FIT-GFR value obtained 2 mo earlier compared the FIT-GFR value obtained from frozen samples stabilized with 10% glycerol (data not shown).
DISCUSSION
FIT Diagnostic Platform
This report presents for the first time a technologically realistic approach to measure GFR in the laboratory setting. The analytic arm of this test relies on FIT. This immunoassay-based approach eliminates all major analytic barriers, because most clinical and research laboratories already have the equipment and trained personnel necessary to run an ELISA. In addition, immunoassay offers high sample throughput and potential automation. The GFR probe used for the test is a known GFR marker that is nonradioactive and is a clinically available contrast reagent (10). Given the sensitivity of immunoassay, the dose required for the FIT-GFR test can potentially be orders of magnitude lower than the required dose for a standard MRI examination.
As important, this report also introduces the general concept of FIT, which is a new diagnostic platform. Conventional immunoassay tests are analog in nature (present or absent), confirming the diagnosis of disease or the presence of a foreign substance. FIT diverges from this convention by designing tests to evaluate and gauge the functional performance of an organ or organ system by sequential measurements of an exogenously administrated compound, thereby allowing the calculation of a kinetic parameter (see Fig. 4). FIT combines the techniques of immunoassay as a readout system with the concept of pharmacokinetics, the introduction of a xenobiotic reagent, to measure the kinetic behavior of an administrated compound or set of compounds. Limits on the rate of clearance or accumulation of the compound as measured in biological material provide the input parameters to calculate a functional index for the system of interest. GFR is one example of a functional parameter that can be accurately measured by FIT.
Fig. 4.
Comparison of conversional immunoassay and FIT.
The results of this study clearly demonstrate that the FIT-GFR test is sensitive for the detection of Gd-DTPA and provides an accurate and reproducible measurement with a high degree of precision. The presence of serum from a range of different mammals did not interfere with the ELISA's performance. The results of this study also demonstrate the feasibility of using this immunoassay-based readout system to measure GFR in humans. Although this study is limited in size, the sample population spans a wide range of GFR values. The results of this study show that the FIT-GFR kit accurately measures GFR, compared with the clinical standard. Figure 2 shows the comparison of GFR values obtained from the UV/P method. The data virtually fall on the line of unity (Fig. 2A), wherein the error is evenly distributed across the mean from high to low GFR values (Fig. 2B). Figure 3 presents the comparison of GFR values obtained from the blood clearance method. Although the same blood sample values used for the UV/P calculation was used for the blood clearance calculation, the bias and precision of the blood clearance method were not as good compared with the UV/P method. This is largely due to errors in measuring the administered dose for each compound that is required for the calculation. For Gd-DTPA, the injected dose is obtained by measuring the syringe before and after injection. Because the total volume of Gd-DTPA was in the range of 1 ml, the injectate can be accurately determined. However, measuring the high specific radioactivity of [125I]iodothalamate in a much smaller volume (∼0.1 ml) subjected this measurement to a greater degree of error. The poorer result obtained by the blood clearance method is largely due to errors associated with measuring the administrated dose of each compound. Nevertheless, even the results of this comparison are good.
At the Gd-DTPA dose used in this study, serum required a dilution of 1:300 and urine samples required a dilution of 1:3,000 to bring the samples within the active range of the standard curve. Because no measurable sample interference was observed with human serum and urine suggests that the administrated dose could be lowered by as much as two orders of magnitude, which in turn would result in a proportional reduction in the dilution factors for serum and urine. In addition, the high level of sensitivity offered by FIT suggests that single-nephron GFR measurements are possible using this technology.
In addition to the ultralow dose of the GFR probe, another advantage of the FIT platform is that it requires only a small amount of sample for processing, i.e., 50 μl of serum or urine or less. As a result, this diagnostic tool can be more easily used to measure GFR in small animal models, such as rodents. Because only a minute amount of sample is consumed by the analysis and because Gd-DTPA is stable in serum and urine samples, samples can be archived and then reassayed at a later date. This is another important advantage for clinical research and drug development. More importantly, because the integrity of the sample does not degrade over time, samples can be collected at a clinical setting and then transported to a remote clinical reference laboratory for analysis. Because Gd-DTPA is a clinically available MRI contrast reagent, the FIT-GFR test offers researchers the ability to use the same diagnostic probe throughout the pharmaceutical development pipeline, i.e., preclinical through human clinical trials.
Measured GFR vs. Estimated GFR
The main advantage of a measured GFR is its accuracy, reproducibility, and reliability. A measured GFR test relies on measuring the renal clearance of an administrated filtration marker under controlled conditions. An ideal filtration marker is a reagent that is freely filtered at the glomerulus, is not bound to plasma proteins, is not reabsorbed, secreted, or metabolized by the tubules, is nontoxic, and is physiologically inert. Therefore, the kinetic clearance of a filtration marker is only influenced by a change in kidney function and will always provide the correct GFR value. As a result, a measured GFR remains the “gold standard” for assessing kidney function (14).
An estimated GFR is derived from evaluating the fluctuation of naturally existing biomarkers. Biomarkers are compounds that exist naturally in the body and can be excreted by the kidney. The accumulation or reduction of these biomarkers in the body can provide an estimate of renal function. Unfortunately, all biomarkers are influenced by a wide range of nonrenal factors that can vary across different patient populations and disease states and can also vary in time, compromising the accuracy of the test. For research applications, biomarkers can vary across different mammalian models. A great deal of effort has been devoted toward the development of correction formulas to improve the diagnostic value of biomarker-based estimates. Although these efforts have attained some level of success, given the large number of variables, both known and unknown, estimated GFR tests are not likely to achieve the high level of accuracy, reproducibility, and reliability associated with a measurement-based test.
Gd-DTPA as a GFR Probe
A major achievement of this report is the demonstration that an ELISA test can be developed for a small-molecular-weight reagent for the purpose of measuring GFR. Although alternative reagents could have been chosen, Gd-DTPA was selected because 1) Gd-DTPA has an excellent safely profile compared with iodinated CT contrast reagents; and unlike inulin, 1) Gd-DTPA is clinically available for human use 3) is not expensive, and 4) is available in more than 100 countries worldwide. As a result, an ELISA-based, Gd-DTPA GFR test could be universally used in most research and clinical settings.
Recently, there have been concerns raised about the general use of gadolinium-containing compounds in patients with severe renal insufficiency, i.e., stage V patients having a GFR of 15 ml·min−1·1.73 m−2 or lower, due to gadolinium's potential association as a trigger for nephrogenic systemic fibrosis (NSF) in this patient subgroup (19). NSF is a systemic fibrosing disorder that was first described in the literature in 2000, with the first reported case going back to 1997 (6). The disease has been reported only in patients with severe renal impairment. The etiology of NSF is still unknown but is likely multifactorial, having a strong link to specific gadolinium-containing contrast reagents.
As of April 2008, the vast majority (91%) of confirmed clinical NSF cases are attributed to the use of one specific gadolinium-containing reagent, gadodiamide (Omniscan; Gd-DTPA-BMA), while in comparison, only 4% of the confirmed cases were associated with Gd-DTPA and 3% of the cases involved patients that were not exposed to gadolinium-containing reagents (5). This disparity cannot be attributed to market share, because Magnevist (Gd-DTPA) outsells Omniscan by nearly 2:1 in the United States (1). The current body of knowledge is largely based on retrospective clinical studies, wherein proposed mechanisms for the root cause cannot be tested. Recently, two basic science investigations have emerged that have tested potential trigger mechanisms under control conditions. Edward et al. (7) showed that the compound Omniscan acts as a growth factor and promotes the growth of fibroblasts. This effect was specific to Omniscan, not free gadolinium, and was dose dependent. Their findings complement a second paper that demonstrates that high doses of Omniscan will induce NSF-like pathology in rats, but high doses of Gd-DTPA will not induce NSF (18). Although the cause of NSF still remains unknown, the link between NSF and gadolinium-containing compounds may prove to be dose dependent and compound specific. Although caution should always be given when regents are administered that clear via the kidneys to patients with severe renal insufficiency, the FIT-GFR test uses an ultralow dose of Gd-DTPA as the GFR probe and is expected to be safe for the vast majority of research applications.
Cost Advantage of FIT-GFR Test
An advantage of the FIT-GFR test over competing analytic methods is cost. The FIT-GFR kit is currently provided as a fixed 96-well plate, wherein 12 wells are needed for the 5 standards and 1 blank that are run in duplicate. Therefore, the cost on a per subject basis is tied to the number of samples generated by the chosen GFR protocol. For example, in the human clinical trial presented above, two different GFR protocols were used. For the UV/P protocol, seven samples needed to be analyzed in duplicate per subject. As a result, six subjects can be processed per plate at a direct cost of $50 per GFR measurement, whereas for the blood clearance protocol, only four samples per subject were needed for analysis to obtain a GFR measurement. Therefore, 10 subjects can be processed per plate at a cost of $30 per GFR measurement. Many researchers advocated the three-point blood clearance method to minimize the number of blood collections. For such a protocol, 14 subjects can be processed per plate, reducing the cost to $21 per GFR measurement. Because only a minute amount of Gd-DTPA (Magnevist) is needed per injection, the probe cost per subject is negligible. Moreover, because most analytic and clinical laboratories routinely run ELISA tests, these facilities already have the equipment, infrastructure, and trained personnel needed to run a FIT test. Therefore, the startup costs are low.
GRANTS
The development of the FIT-GFR kit was in part supported by a Phase I Small Business Innovation Research Grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1-R43-DK-078404-01).
DISCLOSURES
Drs. C. P. Reinhardt, E. V. Groman, and D. E. Vaccaro are stockholders in BioPAL, Inc.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abu-Alfa AK, Weinreb JC. Nephrogenic systemic fibrosis and gadolinium-based contrast agents—etiology and risk management. Ithaca, NY: International Center for Postgraduate Medical Education, 2007. [CME DVD].
- 2.Anderson DJ Determination of the lower limit of detection. Clin Chem 35: 2152–2153, 1989. [PubMed] [Google Scholar]
- 3.Biggi A, Viglietti A, Farinelli MC, Boada C, Camuzzini G. Estimation of glomerular filtration rate using chromium-51 ethylene diamine tetra-acetic acid and technetium-99m diethylene triamine penta-acetic acid. Eur J Nucl Med 22: 532–536, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurements. Lancet 1: 307–310, 1986. [PubMed] [Google Scholar]
- 5.Broome DR Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol 66: 230–234, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 23: 383–393, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Edward M, Quinn JA, Mukherjee S, Jensen MBV, Jardine AG, Mark PB, Burden AD. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol 214: 584–593, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Gera M, Slezak JM, Rule AD, Larson TS, Stegall MD, Cosio FG. Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant 7: 880–887, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Groman EV, Vaccaro DE, Reinhardt CP. Functional immunoassay technology (FIT™) allows the development of diagnostic assays for glomerular filtration rate (GFR) and effective renal blood flow (ERBF) that are accurate and easy to use (Abstract). Oak Ridge Conference, Pushing the Technology Envelope lll: The Next Generations of Diagnostic Testing, Abstract 85, April 20 and 21, 2006, San José, CA.
- 10.Hackstien N, Kooijman H, Tomaselli S, Rau WS. Glomerularfiltration rate measured using Patlak plot technique and contrast-enhanced dynamic MRI with different amounts of gadolinium-DTPA. J Magn Reson Imaging 22: 406–414, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim HN, Rogers T, Tello A, Matas A. The performance of three serum creatinine-based formulas in estimating GFR in former kidney donors. Am J Transplant 6: 1479–1485, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Mariat C, Alamartine E, Afiani A, Thibaudin L, Laurent B, Berthoux P, De Filippis JP, Thibaudin D, Mayor B, Elessawy AB, Berthous F. Predicting glomerular filtration rate in kidney transplantation: are the K/DOQI guidelines applicable? Am J Transplant 5: 2698–2703, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Mariat C, Alamartine E, Barthelemy JC, De Filippis JP, Thibaudin D, Berthoux P, Laurent B, Thibudin L, Berthoux F. Assessing renal graft function in clinical trials: can tests predicting glomerular filtration rate substitute for a reference method? Kidney Int 65: 289–297, 2004. [DOI] [PubMed] [Google Scholar]
- 14.The National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002. [PubMed] [Google Scholar]
- 15.Perico N, Gaspari F, Remuzzi G. Assessing renal function by GFR prediction equations in kidney transplantation. Am J Transplant 5: 1175–1176, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Poge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 5: 1306–1311, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun Hall PM. Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 6: 100–108, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Sieber MA, Pietsch H, Walter J, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol 43: 65–75, 2008. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. FDA news: FDA requests boxed warning for contrast agents used to improve MRI images. Washington, DC: US Food and Drug Administration, May 23, 2007.
- 20.White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA. Estimating glomerular filtration rate in kidney transplantation: a comparison between ser creatinine and cystatin C-based methods. J Am Soc Nephrol 16: 3763–3770, 2005. [DOI] [PubMed] [Google Scholar]
- 21.White CA, Huang D, Akbari A, Garland J, Knoll GA. Performance of creatinine-based estimates of GFR in kidney transplant recipients: a systematic review. Am J Kidney Dis 51: 1005–1015, 2008. [DOI] [PubMed] [Google Scholar]






