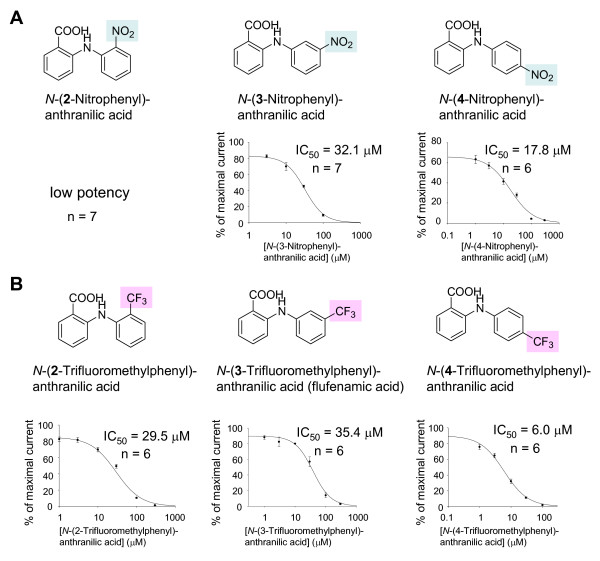
Figure 4.
Positional effect of substituent group on the phenyl ring of blocker that affects block of Ca2+-activated Cl- current. (A) Comparison of chemical structure, IC50 and dose response between N-(2-nitrophenyl)anthranilic acid, N-(3-nitrophenyl)anthranilic acid and N-(4-nitrophenyl)anthranilic acid in which the nitro (-NO2) group on the benzene ring is positioned at ortho, meta and para position. (B) Comparison of chemical structure, IC50 and dose response between flufenamic acid and derivatives N-(2-trifluoromethylphenyl)anthranilic acid and N-(4-trifluoromethylphenyl)anthranilic acid in which the trifluoromethyl (-CF3) group on the benzene ring is positioned at ortho, meta and para position. Shaded boxes indicate the substituent groups tested. n indicates number of oocytes. Error bars indicate SEMs.