Figure 4. Models for the in vitro and in vivo oscillators.
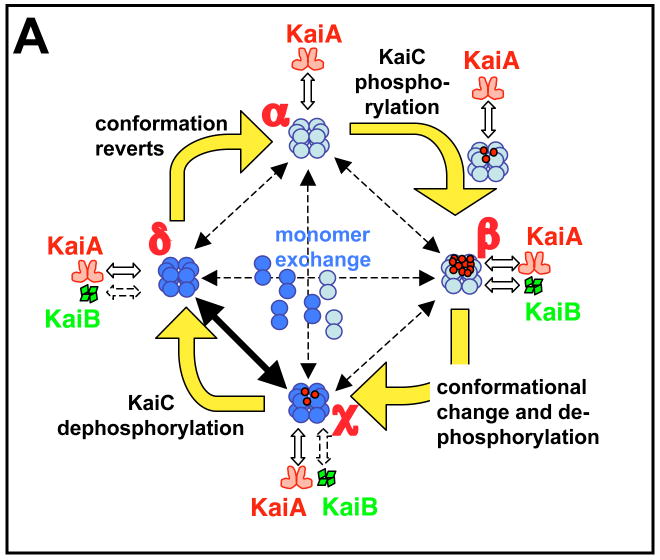
(A) The diagram represents a mathematical model for the phosphorylation cycle of a KaiC hexamer and its association with KaiA and KaiB [56]. A KaiC monomer is shown as a double circle that can form a hexamer. KaiC hexamers can associate/dissociate with KaiA and/or KaiB. KaiC hexamers are depicted in two conformational states: a default KaiC status (light blue color) and an altered KaiC* state that has undergone a conformational change (darker blue color). Red dots are phosphates attached to the S431 and T432 phosphorylation sites on KaiC. Starting from a hypo-phosphorylated state (α), rapid binding and unbinding of KaiA facilitates autophosphorylation until the KaiC hexamers are hyper-phosphorylated (state β). In this model, KaiB is assumed to preferentially associate with hyper-phosphorylated KaiC; there is a simultaneous conformational change of KaiC to a new state (KaiC* depicted as a darker blue hexamer). The KaiC* hexamer de-phosphorylates through states χ and δ until it is no longer phosphorylated, at which time it reverts to the original conformation (state α). Robustness is maintained by synchronization of KaiC hexameric status via monomer exchange [55–57], depicted by “dumbbell” KaiC monomers exchanging with KaiC hexamers in the central region of the figure. The rate of this exchange is highest during the dephosphorylation phase [57], as indicated by the thicker exchange arrow in this phase.
(B) Inhibiting protein synthesis does not perturb the phase of the clock. Cyanobacterial cells were grown in batch culture prior to addition of the translational inhibitor chloramphenicol. Chloramphenicol (50 μg/ml) was applied to cyanobacterial cultures for various durations, then removed. The chloramphenicol treatment began immediately after a 12 h exposure to darkness that synchronizes the cells in the population. Filled circles indicate the phases of the troughs of the rhythm of psbAI promoter activity after wash-out of chloramphenicol. If the clock were stopped or otherwise perturbed by the chloramphenicol treatment, the phase of the subsequent rhythms would be expected to follow the diagonal line of the wash-out treatments. In contrast, we found that the phase of the subsequent rhythms align vertically, indicating that the cyanobacterial clock’s phase was not affected by the chloramphenicol treatments (in the first cycle after chloramphenicol wash-out [~hours 24–36], there was a transient impact upon the phase of psbAIp activity, but thereafter the steady-state phase of the rhythm was unaffected by the chloramphenicol treatment) [38].
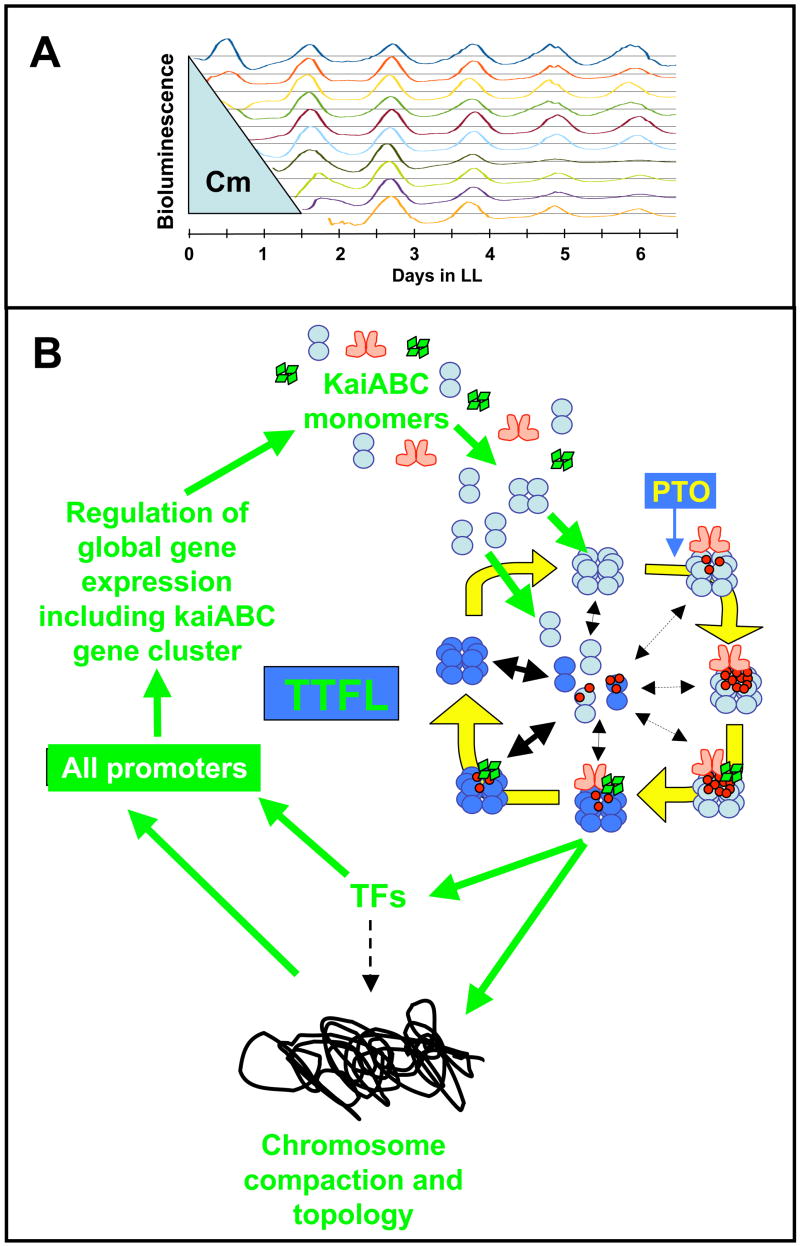
(C) A self-sustained post-translational oscillator (PTO) embedded within a transcription and translation feedback loop (TTFL). The PTO is shown in greater detail in panel A. New synthesis of KaiC monomers feeds into the KaiABC oscillator as non-phosphorylated hexamers (panel C) or as monomers that exchange into pre-existing hexamers (panel C). If the new synthesis of KaiC occurs at a phase when hexamers are predominantly hypo-phosphorylated, the oscillation of KaiC phosphorylation is reinforced (enhanced amplitude). If on the other hand, new synthesis of unphosphorylated KaiC happens at a phase when hexamers are predominantly hyper-phosphorylated, this could lead to an overall decrease in the KaiC phosphorylation status, thereby altering the phase of the KaiABC oscillator (phase shift) and/or reducing its amplitude. Some configuration of KaiC (here shown as the dephosphorylating phase, but it could be any phase of the PTO) mediates rhythmic DNA torsion/compaction and transcriptional factor activity to control global transcription of all promoters, including those driving expression of the essential clock genes kaiA, kaiB, and kaiC (“TFs” = transcriptional factors such as the putative transcriptional factor RpaA).