Abstract
Nitric oxide (NO) released by myenteric neurons in isolated segments of guinea pig ileum was monitored in vitro using continuous amperometry. NO was detected as an oxidation current recorded with a boron-doped diamond microelectrode held at 1 V versus a Ag|AgCl reference electrode. This potential was sufficient to oxidise NO. Longitudinal muscle myenteric plexus (LMMP) and circular muscle strip preparations were used. In the LMMP preparation, NO release was evoked by superfusion of 1 μM nicotine, which activates nicotinic acetylcholine receptors expressed by myenteric neurons and myenteric nerve endings. The oxidation current was ascribed to NO based on the following observations: (i) no response was detected at less positive potentials (0.75 V) at which only catecholamines and biogenic amines are oxidized, (ii) the current was abolished in the presence of the nitric oxide synthase antagonist, N-nitro-L-arginine (L-NNA) and (iii) oxidation currents were attenuated by addition of the NO scavenger, myoglobin, to the superfusing solution. In the LMMP preparation stimulated release produced a maximum current that corresponded nominally to 46 nM of NO. The oxidation currents decreased to 10 and 2 nM, respectively, when the tissue was perfused with tetrodotoxin and L-NNA. Oxidation currents recorded from circular muscle strips (stimulated using nicotine) were 3-fold larger than those recorded from the LMMP. This study shows that NO release can be detected from various in vitro preparations of the guinea pig ileum using real-time electroanalytical techniques.
Keywords: electrochemistry, myentric plexus, nitric oxide, intestine
Introduction
Nitric oxide (NO) is a gaseous signalling molecule that serves diverse functions throughout the body. In the gastrointestinal (GI) tract, NO is an inhibitory neurotransmitter to the muscle layers where it causes relaxation [1,2]. NO is synthesized by the enzyme, nitric oxide synthase (NOS) and the neuronal isoform is found in inhibitory motor neurons and some descending interneurons of myenteric neurons [3,4]. NO can also be synthesized and released by other cell types in the gastrointestinal tract, including smooth muscle, macrophages and other immune cells, and interstitial cells of Cajal [5]. Synthesis and release of NO can be blocked by NOS inhibitors, such as N-nitro-L-arginine (L-NNA). Deficiency in the synthesis or release of NO by myenteric neurons contributes to impaired gastric motility and delayed gastric emptying in animal models of diabetes [6]. Reductions in the number of NOS-containing neurons might also contribute to the pathophysiology of gastroparesis in human diabetic patients [5]. NO is also released by immune cells contributes to gastrointestinal inflammation, which can also alter gut function [6,7].
It is clear that NO is an important signalling molecule in the gut yet there are few techniques that can be used for its real-time measurement near the sites of release. Drug or electrical stimulation induced relaxation of smooth muscle can be used as an indirect measure of NO release from myenteric neurons. However, muscle function can influence the relaxation caused by nerve stimulation, so this bioassay can be influenced by factors (e.g. other inhibitor neurotransmitters such as ATP, and VIP) other than the amount of NO released by motorneurons. There is a need for better techniques that can provide quantitative measurement of NO released by neurons and other cell types in the gut. Improved NO measurements will lead to a better understanding of its role in normal and abnormal gut function.
Electrochemical methods can provide a wealth of information regarding the release and clearance rates of electroactive neurotransmitters as these methods are ideally suited for measurement of transient changes in neurotransmitter concentration near the sites of release. In these methods, the electroactive neurotransmitter is detected as an oxidation or reduction current at a recording microelectrode. The current magnitude is controlled by the flux of the neurotransmitter at the electrode surface. There are two methods typically employed: continuous amperometry (CA) and fast scan cyclic voltammetry (FSCV). Use of both techniques has led to a better understanding of neurotransmission in the brain through the detection of numerous electroactive compounds including dopamine, noradrenaline (NA), 5-HT, acetylcholine, GABA and glutamic acid [8-11]. CA involves applying a fixed potential, with respect to a thermodynamic reference electrode, and measuring the current that flows as a function of time. The potential applied is one at which the neurotransmitter of interest can be electrolyzed at a mass transfer limited rate. The current is controlled by the flux of analyte reaching the electrode and all electroactive species arriving at the electrode contribute to the current. The flux of analyte reaching the electrode over time after exocytosis is dependent in a complex manner on the amount of neurotransmitter released, the rate of release and the rate of clearance. CA is probably the most often used of the two methods. It affords good sensitivity and temporal resolution, but is sensitive to changes in the local ionic concentration and it lacks sufficient selectivity for many applications [8-11]. On the other hand, background-corrected FSCV provides considerable temporal resolution and chemical selectivity. In this method, the potential is scanned over a range of values (e.g., -0.5 to 1.2 V vs. Ag|AgCl reference electrode) and the redox behavior of molecules in the vicinity of the recording microelectrode is probed. If the electroactive neurotransmitters or metabolites possess different oxidation and reduction potentials, then qualitative and quantitative information can be obtained about each. The resulting current-voltage curves, recorded as a function of time, provide a “fingerprint” for the molecules detected at the electrode and reflect transient changes in the local concentration of each [8-11].
To date, there has been limited use of electrochemical methods to study neurosignalling in the gut. In the only reported work, CA was used for real time measurement of serotonin release from enterochromaffin cells in the intestinal mucosa [12-15] and BON cells [16]. These studies have helped to elucidate some of the mechanisms controlling 5-HT disposition. In the present study, we show that electrochemical methods have applicability for studies of intercellular signalling in the gut as we have used CA to monitor locally-produced NO in isolated segments of guinea pig ileum. These measurements can be performed in real time and detection limits for NO are in the low nanomolar range. They provide direct information on NO concentration transients near the sites of release. Since the microelectrodes are small, they can be carefully placed in tissue without causing peripheral tissue damage. NO is electrochemically active at many electrodes and can be detected as an oxidation current that is proportional to the flux of NO at the electrode surface. This flux is contributed to by the rate of production from nearby NOS-containing cells and rate of mass transfer to the recording electrode. Previous work has shown that electrochemical techniques can be used to detect NO release in the brain, from neurons in culture [17-21] and from endothelial cells [21,22]. A challenge for the electrochemical measurement of NO at most electrodes is selectivity because of the positive potential at which the molecule is oxidized. There can be other electroactive compounds present in many tissues including noradrenaline, serotonin and nitrite that could contribute to the current. Furthermore, non-oxidizable compounds (e.g., protein and lipids) can also cause electrode fouling due to adsorption processes and this leads to time dependent attenuation in the electrode response. These problems are often circumvented by coating the electrode with a polymer that is permselective for NO. In this paper, we demonstrate that the boron-doped diamond (BDD) microelectrode, without any permselective polymer coating, provides good sensitivity, selectivity and stability (resistance to fouling) for locally monitored NO transients in the myenteric plexus of the guinea pig ileum. This is the first description of measurements of real-time synthesis and release of NO from myenteric neurons.
Materials and Methods
Tissue preparation
Animal use procedures were approved by the All University Committee on Animal Use and Care at Michigan State University. Male guinea pigs weighing 300-400 g were anesthetized via halothane inhalation, stunned and exsanguinated by severing the major neck blood vessels. A segment of ileum was harvested 15-20 cm proximal to the ileocecal junction and placed in oxygenated (95% O2 and 5% CO2) Krebs' solution of the following composition (millimolar): NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; glucose, 11. The Krebs' solution contained nifedipine (1 μM) to block longitudinal muscle contractions and scopolamine (1 μM) to block muscarinic cholinergic receptors. A 1.5 cm segment of ileum was cut open along the mesenteric border and pinned out flat with the mucosal surface up in a petri dish lined with a silastic elastomer (Dow Corning Co., Midland, MI). For the longitudinal muscle-myenteric plexus (LMMP) preparation, a window of the LMMP preparation was exposed by peeling away the mucosal, submucosal, and circular muscle layers using fine forceps and scissors. For the circular muscle, a similar protocol was applied with a window of circular muscle exposed by peeling away the mucosal and submucosal layers. A 5 mm2 window of either the LMMP or circular muscle in a 2 cm piece of mucosal tissue was then transferred to a 2 mL-volume recording chamber. The preparation was stretched lightly and fixed to the chamber bottom using stainless steel pins (50 μm diameter). The preparation was superfused with a 36 °C oxygenated Krebs' solution at a flow rate of 4 ml min-1.
Electrochemical measurement of nitric oxide
Continuous amperometric (CA) recording of NO production was made from myenteric ganglia using a three-electrode configuration. The NO oxidation current was recorded using a 40 μm diameter boron-doped diamond microelectrode. The procedure for fabricating the electrode has been described elsewhere [13, 23]. NO measurements were also made using a protruding tip 30 μm disc carbon fibre microelectrode with similar apparent area, for comparison. A Pt wire served as the counter electrode and a “no leak” Ag|AgCl electrode was used as the reference (EE009, ESA Biosciences, Inc. USA) electrode. For characterisation measurements, differential pulse voltammetry (a potential step technique in which the current is recorded whilst the potential is ramped in a series of small potential steps) was carried out for characterisation methods and for in vitro recording continuous amperometry (a technique in which the current is recorded with the potential is held constant) was utilised. The reference and counter electrodes were placed in the flow bath but away from the site where a measurement was being conducted. All amperometric measurements were performed using a BioStat™ multi-mode potentiostat (ESA Biosciences, Inc, USA). The boron-doped diamond microelectrode was reproducibly positioned near a myenteric ganglion using a micromanipulator (Model 25033, Fine Scientific Tools, USA). A similar approach was used for measurement on the circular muscle.
The electrode was held at a detection potential of 1.0 V versus a Ag|AgCl reference electrode. Measurements were carried out at 10 Hz, where a box filter was utilised to experimental data using Igor Pro software. The methodology applied to stimulate the tissue was based upon that described previously by Patel et al [19]. Nicotine (1 μM) was applied to the tissue by means of a superfusion pipette, which was placed within 100 μm of the tissue. This evoked NO production by NOS. Tissues were continually perfused with Krebs' buffer and switched to a solution containing nicotine in Krebs' buffer for periods of 20 seconds, before reverting back to perfusion with normal Krebs' buffer. Flow rates from the superfusion pipette were 0.2 mL min-1. The nitric oxide synthase (NOS) antagonist, N-nitro-L-arginine (100 μM L-NNA), was used to inhibit NO production. The sodium channel blocker, tetrodotoxin (0.1 μM TTX), was used to verify the neural dependence of NO release. The NO scavenger, myoglobin (0.05 mg mL-1), was used as an additional pharmacological tool to verify that measured currents were due to NO oxidation.
Data presentation and analysis
The peak oxidation current amplitude was measured for each stimulated response and compared to stimulated oxidation currents measured in the presence of TTX and L-NNA during CA measurements at 1 V. The results are expressed as mean ± SEM with “n” values indicating the number of animals from which preparations were obtained.
Results
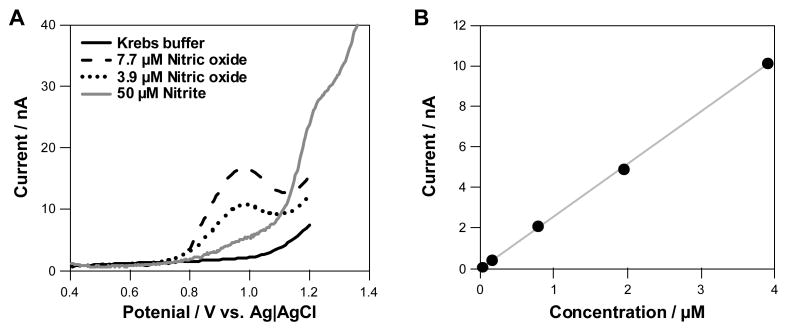
Electrochemical measurements were first performed to verify that NO could be detected oxidatively with a diamond microelectrode and at what potential. Figure 1A shows differential pulse current-voltage curves containing two concentrations of NO, which were produced from a saturated NO aqueous solution (1.93 mM) based upon Henry's Law [24]. NO was introduced into these solutions by adding fixed aliquots of the NO stock solution to a nitrogen-bubbled solution. Relative to the NO-free solution (solid line), it is clear that NO is oxidized based on the concentration-dependent increase in the current amplitude at approximately 0.95 V (dashed and dotted lines). Measurements made in a solution containing NO2- (50 μM) revealed that it does not interfere with the NO measurement as it gets oxidized at a more positive potential (> 1.1 V) [19]. Figure 1B shows a calibration curve for NO recorded with the BDD microelectrode, for which the sensitivity was 2.6 nA μM-1 (r2 = 0.9964). The response variability was 2.6 % at all concentrations for 2 repeat measurements.
Figure 1.
Nitric oxide responses for a boron-doped diamond (BDD) microelectrode using differential pulse voltammetry where the potential was ramped from 0.4 to 1.2 V. Results are shown as potential (x axis) versus oxidation current (y-axis). This method was utilised to determine the potential at which NO gets oxidized and if interferences are present. (A) Current-potential curves for dissolved nitric oxide in Krebs buffer at two concentrations (7.7 and 3.9 μM), Krebs buffer and 50 μM nitrite. The oxidation potential of NO is 990 ± 12 mV and nitrite is 1.2 ± 0.2 V (n = 3). (B) Calibration curve for standard solutions of NO recorded with the BDD microelectrode.
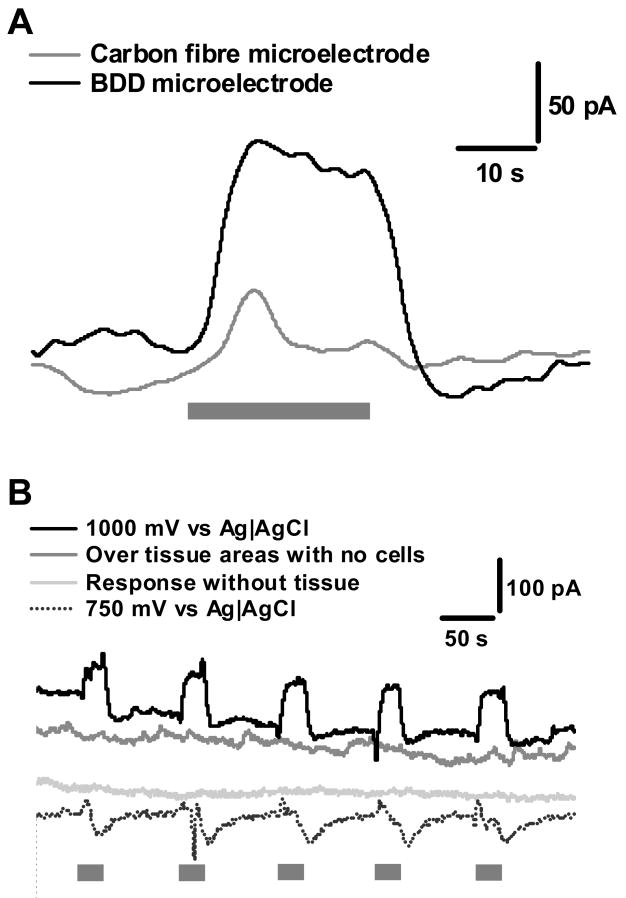
Figures 2 and 3 show a series of CA i-t (current vs. time) recordings of oxidation current in vitro evoked by perfusion of nicotine (1 μM) from LMMP preparation. Experimental controls are shown in Figure 2, whilst biological controls are shown in Figure 3. As observed in Figure 2A, the signal for nicotine-induced NO release is far greater for diamond than for the carbon fibre microelectrode even though the electrode areas were similar. The mass transfer-limited oxidation current is proportional to the geometry and size (e.g., area) of the microelectrode. In Figure 2B, reproducible oxidation currents of approximately 100 ± 13 pA are seen with repeated perfusions of nicotine on the BDD electrode, while the current for the carbon fiber is only about 25 ± 14 pA as shown in Figure 2A (n = 3, p < 0.05). Measurements made in tissue areas outside the myenteric ganglia yielded minimal oxidation current in response to nicotine perfusion. These responses are most likely from NOS containing nerve terminals in the longitudinal muscle. There are no responses observed when measurements are carried out when the tissue is not present. It can also be seen that if the detection potential is lowered to 0.75 V, which is not positive enough to oxidize NO (see Fig. 1A), nicotine does not evoke any current. This suggests that other electroactive substances, such as norepinephrine or serotonin, are not released in response to nicotine stimulation. The dips in the current observed when the measurements were made at 0.75 V may be an artefact caused by changes in the solution ionic composition in the vicinity of the microelectrode caused by the superfused nicotine. The time required to reach the maximum oxidation current from the start of nicotine superfusion was approximately 4 s.
Figure 2.
Experimental controls to show NO detection from myenteric plexus. In all experiments, the grey bar indicates the period during a 20 s superfusion with 1 μM nicotine. This stimulation is provided for all experiments. (A) shows a comparison between a BDD and carbon fibre microelectrode, where both electrodes are held at 1 V. (B) shows the experimental controls, were the electrode is held at a potential to measure NO, when induced using a nicotine stimulation. The controls all show a lack of positive current as would be expected if NO were not present. There are small to no responses when measurements are made without the presence of tissue or in areas outside the ganglionic regions indicating that the majority of release is from NOS containing neurons and terminals from the MP, rather than synaptic terminals within the longitudinal muscle. The trace at 750 mV indicated no interference from catecholamines as no positive deflections in the current were observed.
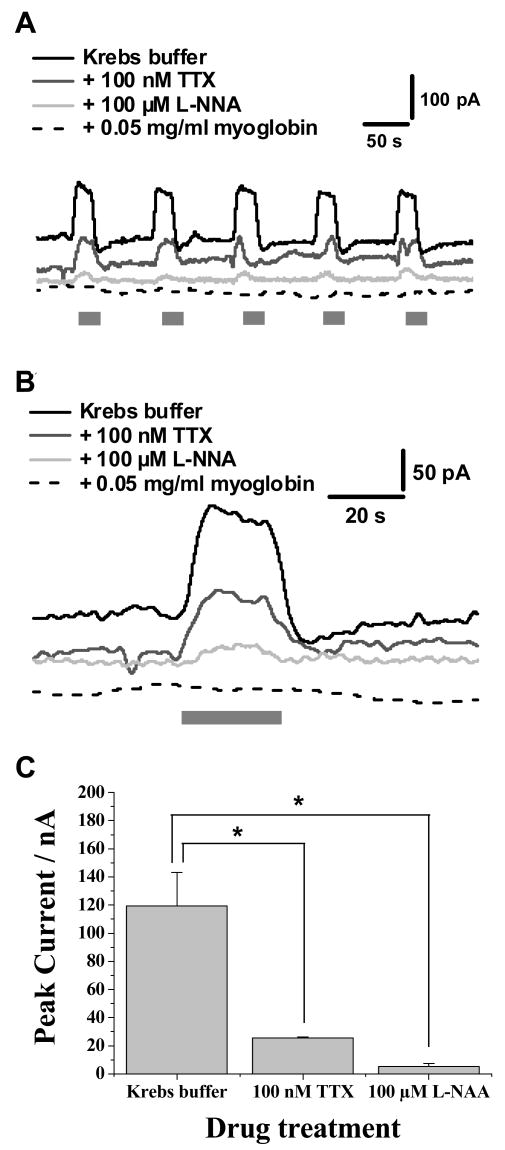
Figure 3.
Detection of nitric oxide from ganglionic regions of the myenteric plexus. In all experiments, the grey bar indicates the period during a 20 s superfusion with 1 μM nicotine. This stimulation is provided for all experiments and only the bath medium is changed when the effects of drug treatments are studied. (A) shows the response elicited from drug treatments, where responses are all carried out at 1 V. Responses in the presence of 100 nM TTX, 100 μM L-NNA and 0.05mg/ml myoglobin are shown. (B) shows an individual response from (A). (C) shows a summary of the changes in nitric oxide levels detected following drug treatments, where results show mean ± SEM, n = 3, *p< 0.001 (paired, two way t-test).
Additional measurements were made to verify that the oxidation current arises from NO. It can be seen in Figure 3 that the oxidation current elicited by nicotine is abolished in the presence of the NOS antagonist, L-NNA. In addition, TTX (100 nM) nearly abolished the current indicating that NO is released almost exclusively by neurons via an action potential-dependent process. In the presence of the NO radical scavenger, myoglobin, the current elicited by nicotine is completely abolished (n = 3). Figure 3B shows an enlarged view of one series of responses for nicotine-induced NO release with and without added L-NNA and TTX. Figure 3C presents a statistical summary of the oxidation current data following pharmacological measurements. Nicotine (1 μM) evoked a maximal current of 119 ± 4 pA (n = 3), which corresponds to approximately 46 nM of NO, as determined from the calculated curve for a series of standards shown in Figure 1B. When TTX or l-NNA were present, the resultant NO oxidation currents decreased to 26 ± 1 pA and 5 ± 2 pA, respectively (n = 3, p < 0.001). These currents correspond to NO concentrations of 10 nM for TTX and 2 nM for L-NNA.
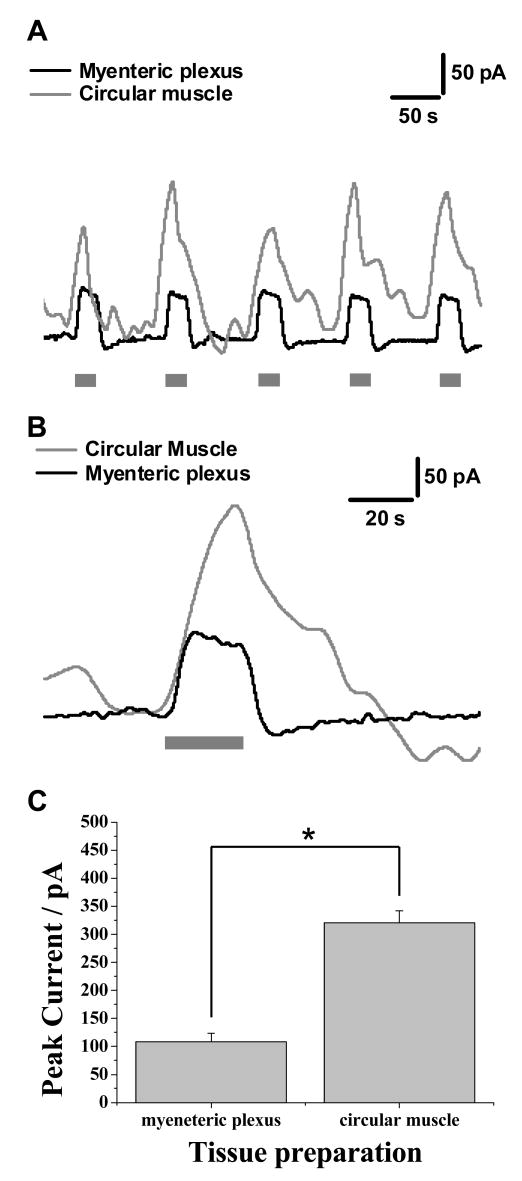
The measurements of NO using the diamond microelectrode described above were obtained from the LMMP preparation. We compared responses from the LMMP with those obtained in strips of circular muscle without the myenteric plexus attached. The circular muscle of the guinea pig ileum contains a dense plexus of NOS-containing nerve fibers. Responses obtained in this tissue are shown in Figure 4, where nicotine evoked a maximal current of 119 ± 4 pA (n = 3) in the myenteric plexus and an increased maximal current of 331 ± 12 pA (n = 3, p < 0.001) from the circular muscle preparation. This corresponds to approximately 124 nM of NO. The shapes of the current transients from the two regions are quite different. For example, the current decay is prolonged in measurements from the circular muscle.
Figure 4.
Comparison of the average data obtained from the myenteric plexus and circular muscle. In all experiments, the grey bar indicates the period during a 20 s superfusion with 1 μM nicotine. (A) shows responses obtained from the circular muscle and myenteric plexus where 5 repeated measurements are obtained. An individual response is shown in (B) and (C) shows a summary of the changes in nitric oxide levels between the two tissue areas. Results show mean ± SEM, n = 3, *p< 0.001 (paired, two way t-test)
Discussion
It has been shown for the first time that electrochemical methods can be used for real-time continuous amperometric measurement of NO release from myenteric neurons of the guinea pig ileum. NOS is expressed by interneurons and motorneurons in the myenteric plexus of the guinea pig ileum [25]. These functional classes of neurons receive fast excitatory postsynaptic input mediated, at least in part, by acetylcholine acting at nicotinic acetylcholine receptors [26, 27]. Therefore, we used bath application of nicotine to activate these neurons. In addition, pharmacological stimulation of neurons eliminates chemical artefacts that might arise from generation of oxygen radicals when currents are conducted through the oxygen-saturated Krebs' solution. However, measurements of NO following electrical stimulation may be achievable, as well as other physiological stimuli, such as gut extension and villi compression. All these stimulation methods are extensively used for studies of the neuronal microcircuitry within the GI tract [28-30].
NO is an important signalling molecule in the gut and most functional studies to date have utilized immunohistochemical or molecular biological techniques to study the localization and expression of NOS under different experimental or pathophysiological conditions. These approaches provide information about the levels of NOS but not its function. Functional approaches have used the response of gut smooth muscle as an indirect measure of NO release from neurons or other cell types. NO is a mediator of inhibitory junction potentials (IJP) and smooth muscle relaxation, and the amplitude of the IJP or relaxation can also be used as an indirect measure of the amount of NO released by neurons in response to various stimuli. While these approaches provide important functional data, they are confounded as the functional properties of the muscle as well as the amount of NO released determine the response. High-performance liquid chromatographic analysis of perfusate collections for levels of l-arginine and l-citrulline is commonly performed to assess NOS activity for NO production [18]. However, this method has two limitations. First, it provides no direct measure of synthesized NO and, second, it provides little temporal information on NO levels.
Responses obtained from the myenteric plexus are obtained from the ganglion regions, where reproducible oxidation currents are obtained. When measurements were carried out in areas between the ganglia, and thus predominately from the longitudinal muscle, minimal currents were observed in response to nicotine stimulation. This would suggest that the majority of the current observed is from the inhibitory neurons from the myenteric plexus during measurements from the LMMP preparation. The probability of measuring NO released from both the terminals in the longitudinal muscle and from cell bodies and terminals of the inhibitory motor neurons of the myenteric plexus is low, due to the high chemical reactivity and short lifetime expected for NO in solution [31,32]. The currents caused by nicotine were completely blocked by NLA indicating the currents were dependent on NOS activity. However, the NLA-sensitive currents caused by nicotine were not completely blocked by TTX as the current was reduced by 80% compared to the control. It is possible that the TTX resistant current is due to NO release from non-neuronal sources including immune cells, smooth muscle or interstitial cells of Cajal [33,34,35]. Alternatively, nicotine could act at nerve terminal nicotinic receptors to activate NOS localized to nerve endings in the muscle. Previous work has shown that myenteric neurons supplying the muscle layers express nerve terminal nicotinic receptors that couple to neurotransmitter release [36].
A key finding from this work is that continuous amperometry (CA) can not only be used to study ganglionic NO signalling but also NO signalling at nerve endings in the circular muscle. These measurements can provide much insight into the mechanisms of physiological release of NO. In many situations, it would be more important to show release from the terminals that are innervating the smooth muscle [28-30]. Detection of NO release from ganglia will not discriminate the sources of the NO production since the NO could be released from cell bodies of either motor neurons or interneurons, or, it could be released from some of NOS-containing terminals within the ganglia [37]. The real time detection of NO from circular muscle preparations is clearly of interest and useful for functional studies of inhibitory motor neurons. Thus, the electrochemical method is a powerful tool for investigating issues related to inhibitory motor neuron and GI functionality.
Continuous amperometry provides information about rapidly changing concentrations of NO near the sites of production independent of smooth muscle or other target cell function. Electrochemical methods provide real-time information on NO with good spatial resolution. Such capability is important for understanding how the expression of nNOS and or the production of NO might be impaired in gastrointestinal disorders. In order to detect NO, there must be selectivity against other interferents that could also be oxidized, such as nitrite, ascorbic acid and catecholamines. In our studies we have accomplished this with a diamond microelectrode and by judicious choice of the detection potential. The amount of 5-HT released from myenteric neurons is low and did not contribute substantially to the oxidation currents measured here. This might not be the case in other tissue beds where interference of catecholamines and biogenic amines is far greater. NO is highly reactive so its lifetime in tissue is short [38]. This leads to rapid and highly localised changes in concentration, typically in the nanomolar to micromolar concentration range. This poses a small problem during biological measurements as the production occurs within the cell, and NO is released in a random fashion by the cell. However if the sensor is localized close to the cell, then direct measurements of release are recorded prior to NO reacting with other constituents in the biological media.
Conclusion
Continuous amperometry with a diamond microelectrode provides a sensitive, stable and selective response for neuronally-produced NO from myenteric neurons and circular muscle in isolated segments of the guinea pig ileum. The method can be used to measure physiological relevant changes in concentration of NO locally within the plexus, specifically, in longitudinal and circular muscle preparations. This new method could prove very useful for assessing impairments in nNOS expression and or NO synthesis and function in gastrointestinal disorders.
Acknowledgments
BAP acknowledges support provided by an EPSRC LSI Postdoctoral Fellowship Grant (EP/C532058/1). This work was also generously supported by the National Institutes of Health HL084258 (GMS) and DK57039 (JJG).
References
- 1.Lecci A, Santicioli P, Maggi CA. Pharmacology of transmission to gastrointestinal muscle. Current Op Pharmacol. 2002;2:630–641. doi: 10.1016/s1471-4892(02)00225-4. [DOI] [PubMed] [Google Scholar]
- 2.Takahasi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 4.Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T. Vagal control of gastric motility: preparation of nitric oxide and vasoactive intestinal polypeptide in the regulation of gastric relaxation. J Physiol. 1995;484:481–492. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 8.Stamford JA. In vitro voltammetry: some methodological considerations. J Neuro Sci. 1986;17:1–29. doi: 10.1016/0165-0270(86)90031-2. [DOI] [PubMed] [Google Scholar]
- 9.Kawagoe KT, Zimmerman JB, Wightman RM. Principles of voltammetry and microelectrode surface states. J Neurosci Meth. 1993;48:225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 10.Travis ER, Wightman RM. Spatio-temporal resoltuion of exocytosis from individual cells. Annu Rev Biophys Biomol Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Troyer KP, Heien MLAV, Venton BJ, Wightman RM. Neurochemistry and electroanalytical probes. Cur Opin Chem Biol. 2002;6:696–703. doi: 10.1016/s1367-5931(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil. 2004;16:511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 13.Patel BA, Bain X, Quaiserova-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 14.Bian X, Patel BA, Dai X, Galligan JJ, Swain GM. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenetrology. 2007;132:2438–2447. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol. 2006;577:689–704. doi: 10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenetrology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Amatore C, Arbault S, Bouret Y, Cauli B, Guille M, Rancillac A, Rossier J. Nitric Oxide Release during Evoked Neuronal Activity in Cerebellum Slices: Detection with Platinized Carbon-Fiber Microelectrodes. ChemPhysChem. 2006;7:181–187. doi: 10.1002/cphc.200500202. [DOI] [PubMed] [Google Scholar]
- 18.Moroz LL, Dahlgren RL, Boudko D, Sweedler JV, Lovell P. Direct single cell determination of nitric oxide synthase related metabolites in identified nitrergic neurons. J Inorganic Biochem. 2005;99:929–939. doi: 10.1016/j.jinorgbio.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Patel BA, Arundell M, Parker KH, Yeoman MS, O'Hare D. Detection of Nitric Oxide Release from Single Neurons in the Pond Snail, Lymnaea stagnalis. Anal Chem. 2006;78:7643–7648. doi: 10.1021/ac060863w. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira NR, Ledo A, Frade JG, Gerhardt GA, Laranjinha J, Barbosa RM. Electrochemical measurement of endogenously produced nitric oxide in brain slices using Nafion/o-phenylenediamine modified carbon fiber microelectrodes. Analytica Chimica Acta. 2005;535:1–7. [Google Scholar]
- 21.Kamei K, Haruyama T, Mie M, Yanagida Y, Aizawa M, Kobatake E. The construction of endothelial cellular biosensing system for the control of blood pressure drugs. Biosensors and Bioelectronics. 2004;19:1121–1124. doi: 10.1016/j.bios.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Oni J, Pailleret A, Isik S, Diab N, Radtke I, Blöchl A, Jackson M, Bedioui F, Schuhmann W. Functionalised electrode array for the detection of nitric oxide released by endothelial cells using different NO-sensing chemistries. Anal Bioanal Chem. 2004;378:1594–1600. doi: 10.1007/s00216-004-2512-6. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Galligan JJ, Fink GD, Swain GM. In vitro continuous amperometry with a diamond microelectrode coupled with video microscopy for simultaneously monitoring endogenous norepinephrine and its effect on contractile response of a rat mesenteric artery. Anal Chem. 2006;78:6756–6764. doi: 10.1021/ac060440u. [DOI] [PubMed] [Google Scholar]
- 24.Battino R, Clever HL. The solubility of gases in liquids. Chem Rev. 1966;66:395–463. [Google Scholar]
- 25.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Takahashi T, Taniuchi M, Hsu CX, Owyang C. Nicotinic receptor mediates nitric oxide synthase expression in the rat gastric myenteric plexus. J Clin Invest. 1998;101:1479–1489. doi: 10.1172/JCI627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PJ, Bornstein JC, Burcher E. Roles of neuronal NK1 and NK3 receptors in synaptic transmission during motility reflexes in the guinea-pig ileum. Br J Pharmacol. 1998;124:1375–1384. doi: 10.1038/sj.bjp.0701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian X, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol. 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian XC, Heffer LF, Gwynne RM, Bornstein JC, Bertrand PP. Synaptic transmission in simple motility reflex pathways excited by distension in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1017–G1027. doi: 10.1152/ajpgi.00039.2004. [DOI] [PubMed] [Google Scholar]
- 31.Philippides A, Husbands P, O'Shea M. Four-Dimensional Neuronal Signaling by Nitric Oxide: A Computational Analysis. J Neurosci. 2000;20(3):1199–1207. doi: 10.1523/JNEUROSCI.20-03-01199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philippides A, Swidbert RO, Husbands P, Lovick TA, O'Shea M. Modeling cooperative volume signalling in a plexus of nitric oxide synthase-expressing neurons. J Neurosci. 2005;25:6520–6532. doi: 10.1523/JNEUROSCI.1264-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vannucchi MG, Corsani L, Bani D, Faussone-Pellegrini MS. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci Lett. 2002;326:191–5. doi: 10.1016/s0304-3940(02)00338-5. [DOI] [PubMed] [Google Scholar]
- 34.Chakder S, Bandyopadhyay A, Rattan S. Neuronal NOS gene expression in gastrointestinal myenteric neurons and smooth muscle cells. Am J Physiol. 1997;273:C1868–75. doi: 10.1152/ajpcell.1997.273.6.C1868. [DOI] [PubMed] [Google Scholar]
- 35.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 36.Schneider DA, Perrone M, Galligan JJ. Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J Pharmacol Exp Ther. 2001;294:363–369. [PubMed] [Google Scholar]
- 37.Bornstein JC. Local neural control of intestinal motility: nerve circuits deduced for the guinea-pig small intestine. Clin Exp Pharmacol Physiol. 1994;21:441–452. doi: 10.1111/j.1440-1681.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 38.Wink DA, Beckman JS, Ford PC. Kinetics of Nitric Oxide Reaction in Liquid and Gas Phase. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. New York: John Wiley & Sons; 1990. pp. 29–37. [Google Scholar]