Abstract
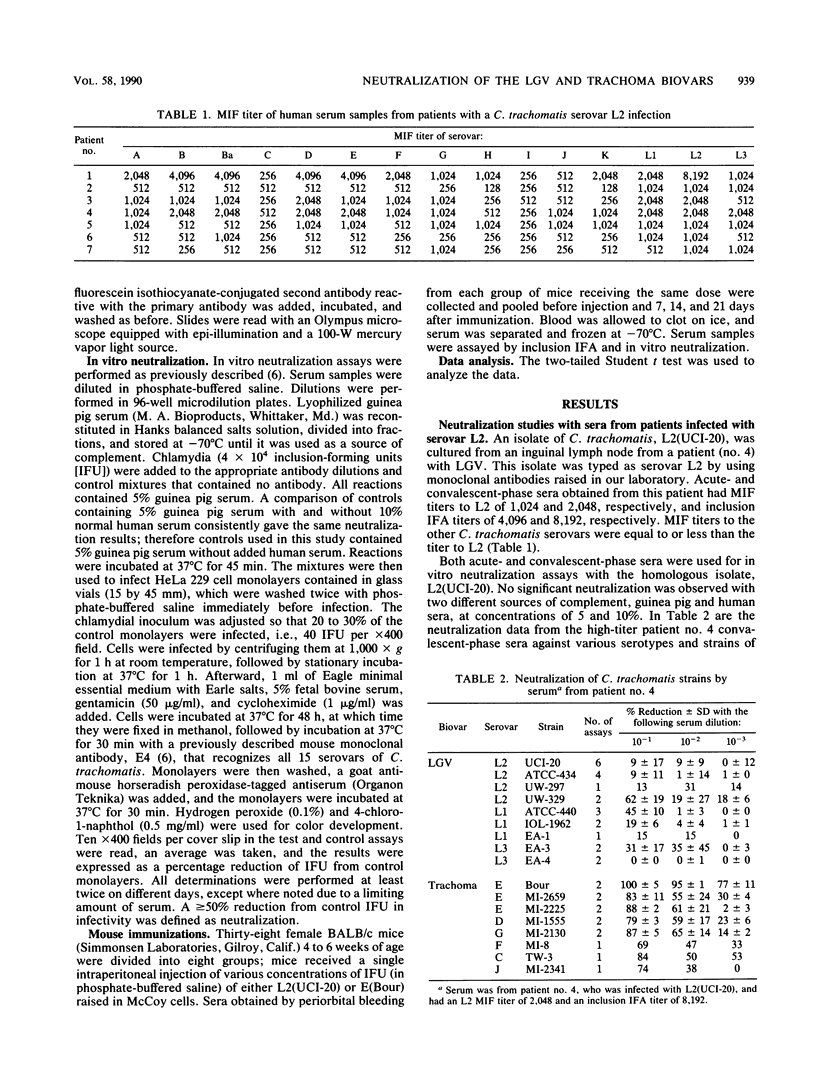
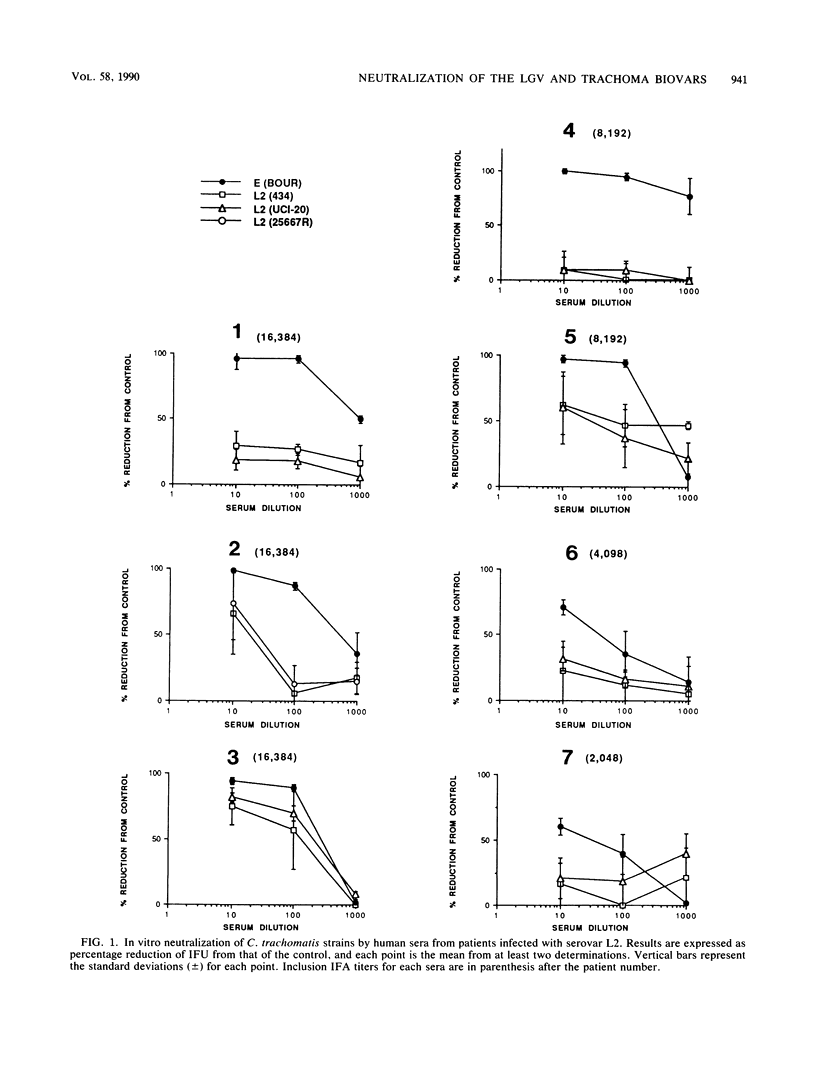
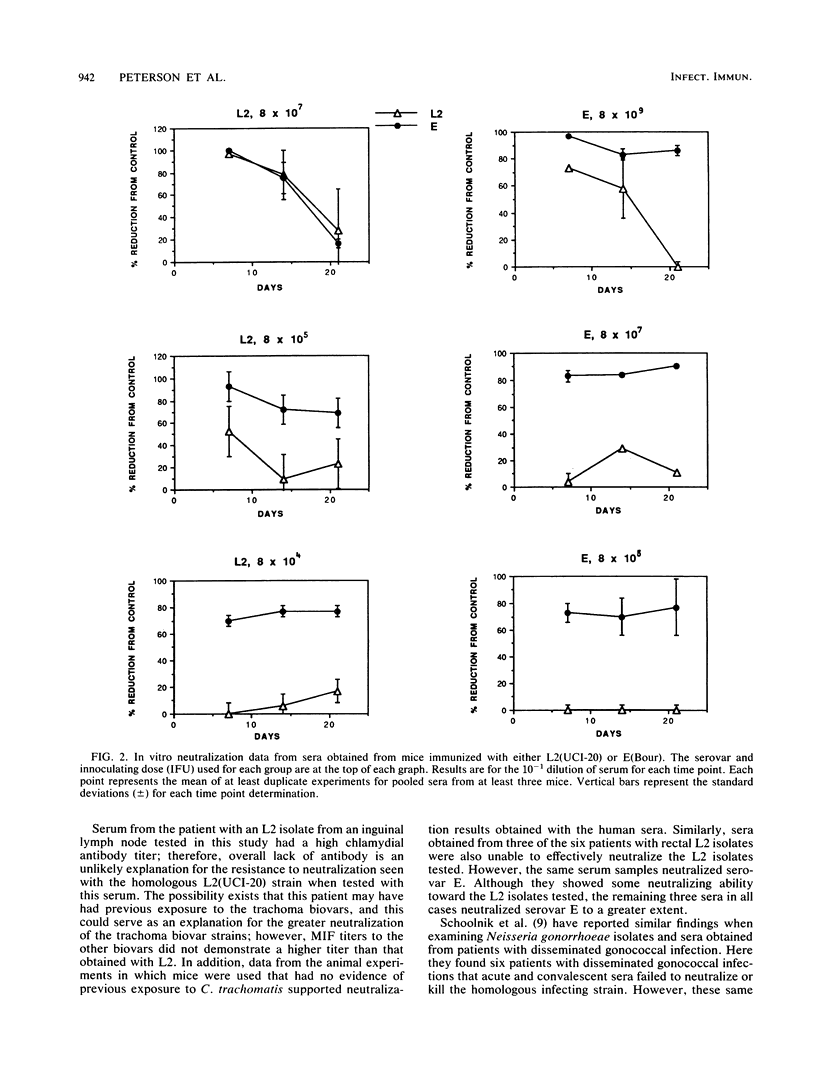
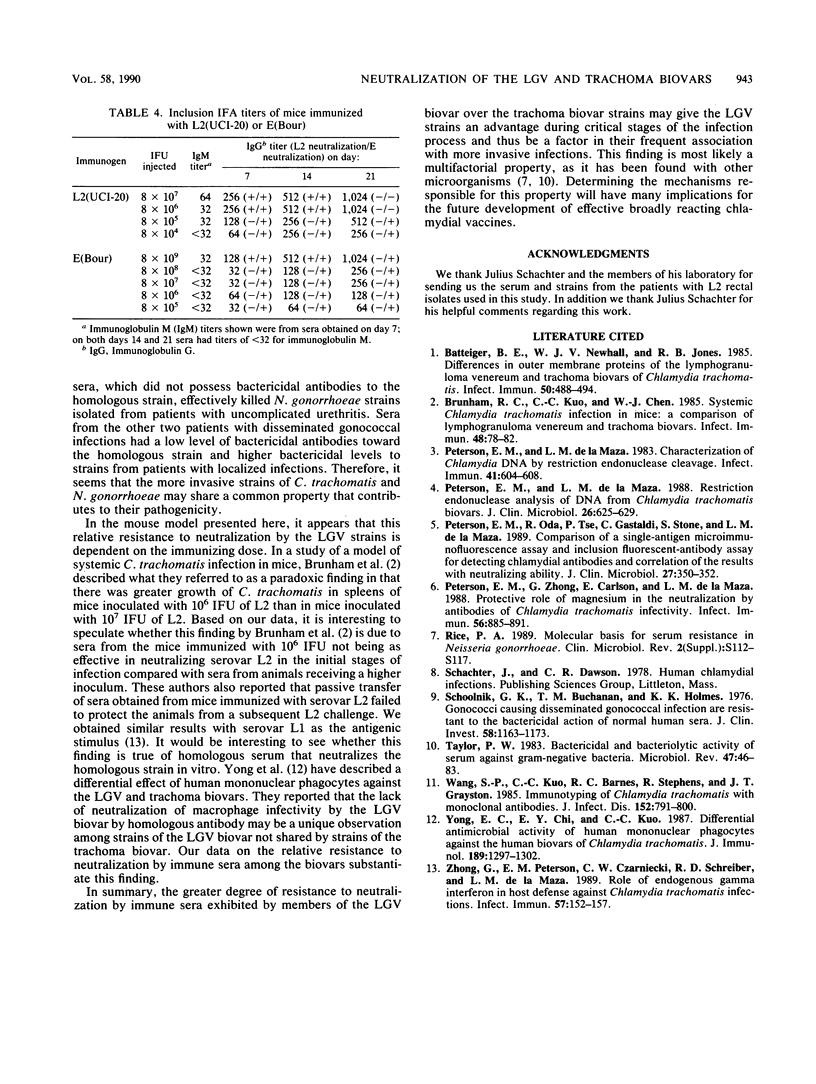
Sera from seven patients from whom a C. trachomatis serovar L2 strain was isolated were tested in vitro for their ability to neutralize the infectivity of this organism. In one patient an inguinal lymph node was culture positive, whereas the remaining six patients had positive rectal biopsies. Sera from four of the patients, including the patient with the lymph node isolate, failed to neutralize serovar L2(434). In addition, the homologous strain recovered from the inguinal lymph node was available and was resistant to neutralization by the homologous sera. However, the same sera effectively neutralized a trachoma serovar, E(Bour). All four sera had inclusion immunofluorescent-antibody titers to C. trachomatis serovar L2 of 2,048 to 16,384 and microimmunofluorescent-antibody titers to the lymphogranuloma venereum biovar were equal or higher in all cases than to the 12 serovars of the trachoma biovar. The three remaining sera, while neutralizing the infectivity of the L2 strains tested, neutralized serovar E to a greater extent. These sera had the same inclusion immunofluorescent antibody titers as the sera that failed to neutralize serovar L2. To see whether this difference in the sensitivity of the biovars toward neutralization could be characterized, sera were obtained from mice immunized with different doses of both serovars L2 and E. Sera obtained from mice immunized with serovar E were able to effectively neutralize the homologous strain. In contrast, neutralization of the immunizing strain, L2(UCI-20), was not seen with sera obtained on days 7, 14, and 21 after immunization from animals receiving 8 x 10(5) and 8 x 10(4) inclusion-forming units of L2(UCI-20); however, these same sera neutralized serovar E. However, with a higher immunizing dose of L2 (10(7) IFUs), both E and L2 were neutralized with sera obtained 7 and 14 days after immunization. Therefore, the relative resistance to neutralization by serovar L2 compared with that of serovar E in the mouse model was inoculum dependent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Kuo C., Chen W. J. Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infect Immun. 1985 Apr;48(1):78–82. doi: 10.1128/iai.48.1.78-82.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Tse P., Gastaldi C., Stone S. C., de la Maza L. M. Comparison of a single-antigen microimmunofluorescence assay and inclusion fluorescent-antibody assay for detecting chlamydial antibodies and correlation of the results with neutralizing ability. J Clin Microbiol. 1989 Feb;27(2):350–352. doi: 10.1128/jcm.27.2.350-352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Restriction endonuclease analysis of DNA from Chlamydia trachomatis biovars. J Clin Microbiol. 1988 Apr;26(4):625–629. doi: 10.1128/jcm.26.4.625-629.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A. Molecular basis for serum resistance in Neisseria gonorrhoeae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S112–S117. doi: 10.1128/cmr.2.suppl.s112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Kuo C. C. Differential antimicrobial activity of human mononuclear phagocytes against the human biovars of Chlamydia trachomatis. J Immunol. 1987 Aug 15;139(4):1297–1302. [PubMed] [Google Scholar]
- Zhong G. M., Peterson E. M., Czarniecki C. W., Schreiber R. D., de la Maza L. M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989 Jan;57(1):152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


