ABSTRACT
BACKGROUND
Many countries have initiated legislation to detect individuals who are unfit to drive, without any evidence that positive effects of these screening procedures outweigh negative effects.
OBJECTIVE
To measure the potential effectiveness of a screening program to detect individuals unfit to drive.
DESIGN
Markov decision analysis was used to compare no screening to two potential screening strategies.
PARTICIPANTS
Hypothetical cohorts of 10,000 45-year-old, 65-year-old, 75-year-old and 85-year-old individuals seen in primary care practices.
INTERVENTIONS
Within the screening strategies: a clinical test without on-road confirmatory testing; a clinical test with on-road confirmatory testing, and an imposed driving cessation for patients with a positive test.
MEASUREMENTS
For each strategy, we compared for two conditions (sleep disorders and dementia) the numbers of crash-related consequences prevented and of adverse events induced (primary objective) and measured the gain in quality-adjusted life years (secondary objective).
RESULTS
For sleep disorders, on-road confirmatory annual testing was the preferred strategy. Whatever the medical condition and age when screening starts, no screening was always better than single-test screening without an on-road confirmatory testing. In sensitivity analyses, these baseline conclusions were only affected by extreme values of test specificity.
CONCLUSION
Because of the expected difficult application and cost of road tests and annual screening by clinicians, the most acceptable strategy from public health, clinical, and individual points of view is likely to be no screening.
KEY WORDS: fitness-to-drive, mass screening, clinical practice, decision analysis
BACKGROUND
Patients with some medical conditions can become unfit for driving, i.e. lack the mental and physical competences required to drive safely1. Screening policies to detect these patients vary dramatically between states or provinces within a same country2–4. Introduction of similar policies is also debated in Europe5. Countries such as the United States, Australia or Canada have implemented procedures to assess fitness to drive, notably at periodical license renewal.2–4 Whereas renewals of licenses are often simple administrative procedures, in other places applicants must perform tests such as vision or road tests when specific medical conditions are present2–4. Individuals who fail the tests may have their driving privileges revoked or restricted.
As driving is an essential component of modern life, and driving restrictions might have unwanted harmful consequences5–7, detection of drivers unfit to drive has become a debated issue8. Of major importance is the definition of the best opportunity to assess the ability to drive and whether physicians would participate on a voluntary or mandatory basis8. Although the usefulness of such programs should be judged by balancing potential positive and negative effect, there is, to our knowledge, no evidence to justify an implication of physicians in such a process.
We designed a decision analysis to compare the effectiveness of three possible strategies to deal with individuals potentially unfit to drive. We applied the decision analysis to sleep disorders and dementia, frequent conditions associated with an increased risk of collision9.
METHODS
A Markov decision model10 was constructed to examine benefits and risks of implementing a program for detecting individuals who are unfit to drive, and analyzed using TREEAGE PRO 2007 (TreeAge Software Inc, Williamstown, MA, USA). Three strategies were evaluated from an individual driver’s perspective and were applied to two conditions: sleep disorders and dementia: 1) no screening, 2) systematic screening by the primary care physician (single test), and 3) systematic screening with a confirmatory on-road test.
Model and Assumptions
Three subtrees were used to model events associated with the strategies (Fig. 1). The probability of each event was based on published literature or assumed, when data were lacking. All relevant probabilities in the Markov cycle were adjusted according to a rhythm of screening set as annual testing or testing every three years.
Figure 1.
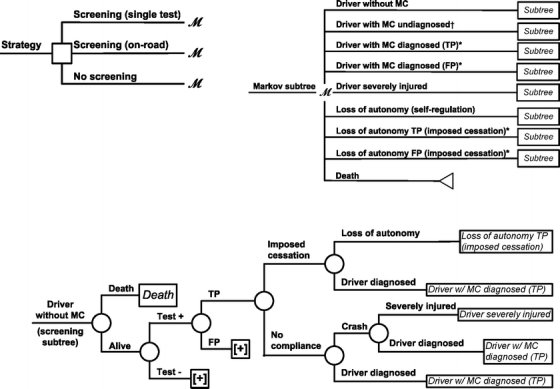
Simplified Markov decision analysis model (for illustrative purposes, only one cycle-tree is shown). *only related to screening; †only related to no screening; MC = medical condition, FP = false positive, TP = true positive; square = decision node, circles = chance nodes, with “M” = Markov nodes; [+] = continuation of tree not shown.
The single-test strategy was defined as testing all individuals not known to have the medical condition, whether they were truly without medical condition, or false negative at previous tests. Data on test sensitivity and specificity was extracted from literature on: the Epworth Sleepiness Scale11, the Multivariate Apnea Prediction (MAP) equation12 or a two-stage approach13 for sleep disorders; the Mini Mental State Examination (MMSE)14,15 or a combination of tests16 for dementia. For the two-test strategy, drivers with a positive first test were referred to on-road confirmatory testing, considered as a gold standard in the literature6,7.
Potential side-effects of an early intervention imposed on all patients with true and false positive tests were considered. Once a patient was positive, there was no return to testing. For patients with true or false negative tests, we considered potential self-regulation strategies and their potential negative consequences. The no-screening strategy corresponds to the natural history of being a driver with or without the medical condition. The number of states in the Markov process was eight for the two screening strategies, and five for no screening.
The following were the baseline assumptions: 1) As done in published decision analyses15,16, all individuals began with full driving privileges as “drivers without medical condition”. 2) The medical condition was irreversible. 3) Compliance with the intervention, defined as a complete cessation of driving, was perfect. 4) Self-regulation strategies adopted by patients with the medical condition were also defined as a complete cessation of driving. 5) In the absence of data on self-regulation strategies adopted by drivers with a medical condition17, a driver without a medical condition would not adopt self-regulation strategies. 6) Side-effects of driving cessation (whether related to self-regulation or imposed) were defined as a loss of autonomy and its consequences. 7) Negative consequences of a crash for individuals submitted to screening (excluding other road users) were defined as a severe injury (person hospitalized at least during 24 hours, a standard definition in European countries18). 8) To take into account long-term morbidity, drivers entering “severely injured” or “loss of autonomy” states (whether related to self-regulation or imposed driving cessation) could die from injury or consequences of the loss of autonomy, or remain in these states. To model morbidity related to the loss of autonomy (such as institutionalization for dementia, or related to depression for drivers aged 45 years old), we increased the risk of death in these states. 9) Because published data were lacking, the probability of side effects from the cessation of driving was set conservatively equal to the probability of crash-related consequences. 10) For sleep disorders, all probabilities except mortality were constant over time; for dementia, prevalence and incidence were varied with time. Data used in analyses are reported in Table 1.
Table 1.
Estimates Used for the Decision Analysis on Detecting Individuals Unfit to Drive*
| Variable | Baseline value (%) | Range (%) | References |
|---|---|---|---|
| Medical condition | |||
| Prevalence of sleep disorders | 2.0 | 0.3–4.0 | 9 |
| Annual incidence of sleep disorders | 3.0 | 0.2–3.5 | Assumed |
| Prevalence of dementia | |||
| 65–69 years of age | 1.0 | 1.0–12.0 | 19 baseline value; 20,21 for range |
| 70–74 years of age | 2.5 | 3.0–12.0 | |
| 75–79 years of age | 6.7 | 6.0–18.0 | |
| 80–84 years of age | 14.5 | 12.0–33.0 | |
| 85+ years of age | 31.1 | 21.0–47.0 | |
| Annual incidence of dementia | |||
| 65–69 years of age | 0.5 | 0.0 - 1.0 | Data from cohort Paquid, 10-year follow-up (NP) for baseline value; 21–23 for range |
| 70–74 years of age | 0.6 | 0.0–2.0 | |
| 75–79 years of age | 1.9 | 1.0–3.0 | |
| 80–84 years of age | 3.1 | 3.0–6.0 | |
| 85+ years of age | 4.5 | no range | |
| Self-regulation strategies (proportion of drivers who stop driving) | |||
| Drivers without medical conditions | 0.0 | No range | Assumed |
| Drivers with sleep disorders | 36.0 | 0.2–63.0 | 24 |
| Drivers with dementia | 12.0 | 0.0–15.0 | Assumed from Paquid (NP) |
| Loss of autonomy related to self-regulation | |||
| 40–59 years of age | 4.7 | 4.7–36.0 | Assumed equal to severe injury |
| 60–79 years of age | 6.2 | 6.2–48.0 | |
| 80+ years of age | 8.3 | 8.3–48.0 | |
| Relative rate of loss of autonomy related to imposed driving cessation (TP and FP) | 1 | 1.7–8.6 | From Paquid (NP) 25 |
| Probability of compliance to intervention | 1 | 0.5–1.0 | Assumed |
| Crash | |||
| Rate of crash of drivers without MC | 4.3 | 0.0–10.0 | 15, and assumed |
| Relative rate of crash in drivers with sleep disorders | 2.0 | 1.5–15.0 | 24,26–29 |
| Relative rate of crash in drivers with dementia | 2.0 | 1.0–5.0 | 15 |
| Rate of crash (drivers without MC and with self-regulation) | 0.0 | 0.0–10.0 | Assumed |
| Relative rate of crash in drivers with MC and self-regulation | 0.0 | 0.0–15.0 | Assumed |
| Rate of severe injury related to crash | |||
| 40–59 years of age | 4.7 | 4.7–36.0 | 18 French national data |
| 60–79 years of age | 6.2 | 6.2–48.0 | |
| 80+ years of age | 8.3 | 8.3–48.0 | |
| Death | |||
| Rate of death related to crash | |||
| 45–65 years of age | 5.0 | 5.0–10 | 15,18 French national data and assumed |
| 65+ years of age | 10.0 | 10–20 | |
| Rate of death related to loss of autonomy | |||
| 45–65 years of age | 5.0 | 5.0–10 | Assumed equal to death related to crash |
| 65+ years of age | 10.0 | 10–20 | |
| Rate of death related to all other causes | Life table | No range | 30 |
| Screening tools | |||
| Sensitivity of questionnaires for sleep disorders | 88.0 | 88.0–91.0 | 13,26 |
| Specificity of questionnaires for sleep disorders | 55.0 | 55.0–85.0 | 13,26 and assumed |
| Sensitivity of MMSE or combination of tests | 85.0 | 46.0–95.0 | 14–16 |
| Specificity of MMSE or combination of tests | 85.0 | 39.0–95.0 | 14–16 and assumed |
* MC indicates medical condition; NP not published; TP true positive; FP false positive
Outcomes
The main objective was to estimate and compare the number of severe injuries and related deaths that would be prevented (advantages), and the number of losses of autonomy and related deaths that would be induced (risks). We tracked variables into the Markov processes to count events (severe injury and related death as “crash-related consequences”, and loss of autonomy and related death as “adverse events”) with an individual Monte Carlo simulation. The numbers of expected events were estimated per 1,000 drivers seen in a primary care practice and simulated over a period of five years.
In a secondary objective, we assigned utilities to all states, estimated from a driver’s perspective (appendix and Table 2), and calculated quality-adjusted life years (QALYs) using Markov cohort analyses. For sleep disorders, a hypothetical cohort of 10,000 45-year-old individuals seen in primary care practices was simulated over five and 10 years. For dementia, age of entry in the cohort was 65 years of age, 75 years of age or 85 years of age .
Table 2.
Utilities Used in the Analysis, Survey of Drivers in an Academic Institution, Bordeaux, France ( = 55)*
| Utilities † | Baseline value (median) | Inter-quartile range (IQR) | Min - max |
|---|---|---|---|
| Driver without MC | 1.0 | 1.0–1.0 | 0.8–1.0 |
| Driver with MC undiagnosed | 0.2 | 0.1–0.4 | 0.0–0.8 |
| Driver with MC diagnosed (TP) | 0.5 | 0.3–0.6 | 0.0–1.0 |
| Driver wrongly diagnosed with MC (FP) | 0.3 | 0.1–0.5 | 0.0–0.9 |
| Driver severely injured | 0.1 | 0.0–0.2 | 0.0–0.4 |
| Loss of autonomy related to self-regulation strategies | 0.4 | 0.3–0.7 | 0.0–1.0 |
| Loss of autonomy in TP, imposed cessation | 0.3 | 0.2–0.4 | 0.0–0.8 |
| Loss of autonomy in FP, imposed cessation | 0.1 | 0.0–0.3 | 0.0–0.9 |
| Death | 0.0 | 0.0–0.0 | 0.0–0.5 |
* MC indicates medical condition; TP true positive; FP false positive; min minimum; max maximum
† Utility between 0 (worst conceivable state) and 1 (best conceivable state); see appendix for full definition of each state
Sensitivity Analyses
One-way and probabilistic Monte Carlo sensitivity analyses were performed to assess whether variables could affect the choice of the strategy. For one-way sensitivity analysis, variables varied discretely across intervals defined in Table 1. To take into account uncertainty regarding the nature of the intervention (limited rather than full driving restrictions), the level of compliance, the frequency of self-regulation strategies, the probability of side-effects and the performance of tests, we run 10,000 Monte Carlo simulations with random selection of values from uniform distributions31, according to ranges defined in Table 1, except for test specificity which varied up to one.
RESULTS
Baseline Analyses
The number of crash-related consequences was always smaller in single-test screening than in other strategies, both for annual testing and testing every three years (Table 3), but this option was associated with more adverse events. For dementia, whatever the age when annual screening started, the number of induced adverse events would always exceed that of crash-related consequences prevented. Screening 1,000 85-year-old drivers seen in primary care practices every three years for dementia would prevent 586 crash-related consequences against 333 adverse events induced. Compared to no screening, a confirmatory on-road testing every three years would prevent 569 crash-related consequences against 270 adverse events induced, making this strategy the preferred option in that group. On-road confirmatory annual testing was also the preferred option for sleep disorders, with a number of crash-related consequences prevented exceeding that of induced adverse events (10 against four per 1,000 drivers).
Table 3.
Numbers* of Crash-Related Consequences and Adverse Events Associated with Each Strategy
| Annual testing | Testing every 3 years | |||||
|---|---|---|---|---|---|---|
| Screening (w/ on-road) | No screening | Screening (single-test) | Screening (w/ on-road) | No screening | Screening (single-test) | |
| Sleep disorders† | ||||||
| Crash-related consequences | 29 | 39 | 16 | 135 | 136 | 89 |
| Adverse events | 55 | 51 | 460 | 35 | 25 | 388 |
| Dementia (age when screening starts) | ||||||
| 65 years of age | ||||||
| Crash-related consequences | 34 | 37 | 30 | 349 | 359 | 208 |
| Adverse events | 21 | 4 | 570 | 158 | 54 | 395 |
| 75 years of age | ||||||
| Crash-related consequences | 46 | 73 | 26 | 297 | 393 | 203 |
| Adverse events | 56 | 10 | 650 | 349 | 108 | 557 |
| 85 years of age | ||||||
| Crash-related consequences | 56 | 115 | 35 | 206 | 775 | 189 |
| Adverse events | 179 | 6 | 630 | 408 | 138 | 471 |
* Numbers of events by 1,000 drivers at 5 years, results provided by Monte Carlo individual simulations
† Screening starts at 45 years of age
Whatever the medical condition, the rhythm of testing, and age when screening starts, no screening was always better, in terms of QALYs, than single-test screening (Table 4). The loss in QALYs was five years for annual testing of sleep disorders. Single-test screening was preferred to no screening for dementia, only in the group of 85-year-old drivers tested every three years, with a five-month gain in QALYs. Adding a confirmatory on-road test (annual testing) was always preferred to no screening for sleep disorders, with a gain in QALYs ranging from two (at five years) to eight months (at 10 years). In the group of 85-year-old individuals tested every three years for dementia, adding a confirmatory on-road test was preferred to the single-test strategy, with a gain in QALYs always lower than one month.
Table 4.
QALYs* Associated to Each Strategy in Drivers with Visual Impairment; Sleep Disorders or Dementia
| Strategy | Annual testing | Testing every 3 years | ||
|---|---|---|---|---|
| At 5 years | At 10 years | At 5 years | At 10 years | |
| Sleep disorders† | ||||
| No screening | 4.662 | 8.706 | 4.017 | 6.627 |
| Screening (single test) | 2.552 | 3.832 | 2.363 | 3.129 |
| Screening w/ confirmatory on-road test | 4.850 | 9.378 | 2.933 | 4.415 |
| Dementia (age when screening starts) | ||||
| 65 years of age | ||||
| No screening | 4.747 | 8.933 | 4.162 | 6.888 |
| Screening (single test) | 3.728 | 5.753 | 3.300 | 4.513 |
| Screening w/ confirmatory on-road test | 4.006 | 6.600 | 3.540 | 5.044 |
| 75 years of age | ||||
| No screening | 4.286 | 7.391 | 3.137 | 4.337 |
| Screening (single test) | 3.492 | 5.118 | 2.775 | 3.402 |
| Screening w/ confirmatory on-road test | 3.746 | 5.763 | 2.936 | 3.674 |
| 85 years of age | ||||
| No screening | 3.094 | 4.489 | 1.385 | 1.565 |
| Screening (single test) | 2.853 | 3.742 | 1.799 | 1.937 |
| Screening w/ confirmatory on-road test | 3.002 | 4.020 | 1.838 | 1.984 |
* Results provided by hypothetical Markov cohort analyses of 10,000 drivers
† Screening starts at 45 years of age
Sensitivity Analyses
For sleep disorders, on-road testing every three years became the preferred strategy for a specificity of at least 73% and a crash rate for drivers without medical condition twice the baseline estimate (Table 5). The baseline conclusion to prefer no screening to single-test screening was mainly affected by the specificity of the tests, but threshold values were most often larger than the maximum possible value (Table 1). On-road testing in 65-year-old or 75-year-old drivers never became the preferred option. Baseline decisions were never affected in the group of 85-year-old individuals tested for dementia.
Table 5.
Variables Affecting the Choice of Preferred Strategies, One-Way Sensitivity Analyses
| Strategy | Variables* | Baseline values | Range of values in favor of compared strategy | ||
|---|---|---|---|---|---|
| Preferred in baseline analysis | Compared | Annual testing | Testing every 3 years | ||
| Sleep disorders | |||||
| No screening | Single-test screening | ||||
| Specificity | 0.55 | 0.97–1.00 | 0.94–1.00 | ||
| No screening | On-road screening | ||||
| Specificity | 0.55 | N/A‡ | 0.73–1.00 | ||
| Rate of crash for drivers without MC | 0.11 | N/A | 0.20–0.26 | ||
| Dementia (age when screening starts) | |||||
| 65 years of age | |||||
| No screening | Single-test screening | ||||
| Specificity | 0.85 | None† | 0.99–1.00 | ||
| 75 years of age | |||||
| No screening | Single-test screening | ||||
| Specificity | 0.85 | None | 0.95–1.00 | ||
*FP: false positive, MC: medical condition
† Choice of baseline strategy never changed
‡ Not applicable
For sleep disorders, the baseline decision was affected in 62% of the simulations (Table 6). On-road confirmatory testing (every three years) became the preferred strategy for a specificity of at least 77% (included in the literature range) and a crash rate for drivers without medical condition almost twice the baseline estimate. For dementia (65-year-old and 75-year-old), the percentage of changing no screening to single-test screening (every 3 years) ranged from 6% to 30% with an associated specificity of 98%, larger than the maximum value of 95% reported in the literature.
Table 6.
Variables Affecting the Choice of Preferred Strategies, Probabilistic Monte Carlo Sensitivity Analyses*
| Strategy | Variables† | Baseline values | Monte Carlo simulations ( = 10,000) | ||
|---|---|---|---|---|---|
| Preferred in baseline analysis | Compared | Threshold‡ | % of simulations affecting baseline decision | ||
| Sleep disorders | |||||
| No screening | Single-test screening | ||||
| Annual testing | Specificity | 0.55 | 0.95 | 4 | |
| Testing every 3 years | Specificity | 0.55 | 0.95 | 12 | |
| No screening | On-road screening | ||||
| Testing every 3 years | Specificity | 0.55 | 0.77 | 62 | |
| Rate of crash for drivers without MC | 0.11 | 0.17 | |||
| Dementia (age when screening starts) | |||||
| No screening | Single-test screening | ||||
| 65 years of age | Testing every 3 years | Specificity | 0.85 | 0.98 | 6 |
| 75 years of age | Testing every 3 years | Specificity | 0.85 | 0.98 | 30 |
* 10,000 simulations
† FP: false positive, MC: medical condition
‡ Values with 90% probability of changing baseline decision
DISCUSSION
For sleep disorders, annual on-road confirmatory testing of drivers 45 years and older seen in primary care practices was the preferred strategy. Whatever the medical condition and age when screening would start, no screening was better than single-test screening, except for dementia in the group of 85-year-old individuals. In sensitivity analyses, these baseline conclusions were mainly affected by extreme thus unlikely values of test specificity.
The use of a Markov model, efficient to model events recurrent over time32, was useful to model the recurrence of testing, or the possible recurrence of being involved in a crash. Moreover, our model can easily be generalized to other age groups, strategies, interventions and medical conditions other than sleep disorders and dementia. This is in contrast with the literature where most studies are focused on strategies limited at older drivers with cognitive impairment15,16,33.
Input parameters were often inaccurately described or lacking in the literature, especially for utilities used in our secondary objective. Utilities should not only represent quality of life but also its relationship with being able to drive or not. Utilities estimated in a sample of patients with the condition or in their relatives would have better face validity. Consistency of our conclusions, however, was tested by sensitivity analyses dealing with the uncertainty of probabilities and utilities, described as plausible distributions rather than discrete values31.
Our model took into account adverse events of imposed driving cessation, already mentioned in previous cost-benefit analyses of screening older drivers, but never analyzed15,16. Nevertheless, we lacked data on this parameter and assumed that the probability of adverse events would equal that of severe injury. In sensitivity analyses, conclusions of the baseline analysis were not affected by this assumption. We also assumed that a negative consequence of driving cessation would result from a loss of autonomy. The relation between lack of autonomy and the prevalence of institutionalization in patients with dementia or cognitive impairment is well documented in the literature34,35. In patients with other conditions, risks associated with driving cessation could be an increase of depressive symptoms or a decrease of out-of-home activity levels, as suggested by Marattoli et al.25,36. For instance, a 45-year-old driver can be precipitated into depression or isolation because of losing his job, due to the imposed driving cessation. Our model is general enough so that such other consequences could be analyzed, provided adequate data are available.
We also tested robustness of decisions by varying parameters for which the uncertainty was the greatest. The most important parameter was test specificity, but the single-test screening strategy was preferred only for extremely high values. Indeed, minimizing the number of false positives is important, given that the risk of crash-related consequences (mostly an issue for true positive) is likely to be lower than the risk of potential adverse consequences (an issue for all positive drivers).
Beside driving cessation, proposed interventions can be license restrictions (limitations of geographic area, time of day, or type of road)37, educational programs38, or treatment of the medical conditions, for instance for patients with sleep disorders39,40. Modeling a complete driving cessation is relevant as it is included in existing policies2–4, and there are major uncertainties regarding effectiveness of alternative interventions. Nevertheless, the effect of license restrictions was indirectly considered in sensitivity analyses, by having compliance being able to be less than 100%.
Unlike published decision analyses15,16, we did not consider costs. We can hypothesize that overall costs might be high for single-test screening, as the loss of autonomy and morbidity induced by imposed driving cessation would imply new needs for managing these consequences, or that benefits of on-road confirmatory testing might be smaller than costs, as this test is time-consuming and expensive5. We also believe that it is too early to carry satisfactory economic evaluations. Indeed, as suggested for instance by Drummond et al41, economical analyses are not relevant in the absence of sufficient evidence regarding the effectiveness of compared interventions. Moreover, a full assessment of costs would imply to define each screening strategy more thoroughly, especially regarding what would be a full intervention (including referral modalities by the clinician, organization of alternative modes of transportation for drivers denied driving privileges, health care for complications of the loss of autonomy...).
The feasibility of on-road testing for dementia has been questioned, especially for drivers aged 85 years and older42. Because driving is an over-learned task, standard road tests with step-by-step instructions do not necessarily test the skills of experienced drivers, nor reveal common errors. While it may be difficult to incorporate challenging driving situations into on-road evaluations, a greater level of difficulty may be needed to obtain a valid assessment of competency, particularly in experienced drivers with cognitive impairment42. The only screening strategy that may be feasible and effective in real conditions would be the single-test screening strategy for dementia, targeted at drivers aged 85 years and older seen in a primary care practice. However, in the absence of valid diagnostic tools, there are multiple barriers to formal assessment of driving competences of drivers with dementia in primary care settings. Indeed, as demonstrated by Boustani et al.43,44, primary care patients’ acceptance about dementia screening is strongly related to the impact on patients’ independence, which is particularly related to driving privileges.
Nevertheless, a recent study indicated that a medical contact, associated with an increased risk of crash in a population of older drivers from Quebec, could represent an opportunity to detect drivers potentially at risk45. However, the acceptability and feasibility for the primary care physician to use a battery of tests to detect inability to drive in all patients of various ages are likely to be low. Targeting a more restricted population with an increased risk could be more acceptable and feasible. For instance, tests for sleep disorders could be applied to those with consistent complaints (inappropriate sleep). Another key issue is to define what would be the role of the physician in managing unwanted consequences induced by the program.
From the targeted population point of view, acceptability of the process is also of major importance: acceptability of being discriminated as member of the group targeted by the strategy, acceptability of tests, acceptability of being false positive, acceptability of imposed driving cessation, acceptability of related loss of autonomy and associated needs to use other modes of transportation... Obviously, potential negative consequences of the program would be even less acceptable for drivers wrongly diagnosed unfit to drive. Finally, acceptability of individuals would have a major impact on compliance to the imposed intervention and thus on the potential effectiveness of the screening strategy.
CONCLUSION
Because of the expected difficult application and cost of road tests and annual screening by clinicians, the most acceptable strategy from public health, clinical, and individual points of view is likely to be no screening.
Aknowledgements
This work was done as part of the PhD thesis of the first author, directed by the last author. The authors thank the Direction de la sécurité et de la circulation routières, Ministère des transports, de l’équipement, du tourisme et de la mer for the funding of this PhD thesis. SL and LRS contributed equally to the elaboration of the Markov Model, evaluation of the literature, drafting and critical revision of the manuscript. EL critically assessed the decision analysis presented in the manuscript, at several stages of its development. SL, EL and LRS read and approved the final version of the manuscript. We would like to acknowledge Karine Perez (INSERM, U897, équipe Épidémiologie et Neuropsychologie du vieillissement cérébral) and Wassilios Meissner (University Hospital Bordeaux, France, Department of Neurology) for their help in data acquisition about natural history of dementia and sleep disorders respectively, and the related-consequences on driving competences.
Conflict of Interest None disclosed.
Appendix
Estimating utilities for the secondary objective
Because no literature was available for utilities expressing both quality of life associated with health states and its relationship with being able to drive or not, we conducted a survey of drivers selected among personal at our institution. Although these estimations were based on a selected sample of drivers, thus could lack face validity, we considered resulting estimates to be an appropriate basis for a secondary objective and to define ranges for Monte Carlo simulations.
We contacted all personal of our institution by E-mail to explain the objective of this survey, along with instructions and a questionnaire, including a description of all states of the model:
Driver without medical condition: “You are an active, safe and able driver.”
Driver with undiagnosed medical condition: “You have a medical condition potentially dangerous for driving; you are not aware of this, because this medical condition has not been diagnosed; you are still an active driver and at higher risk of crash, because of this undiagnosed medical condition.”
Driver correctly identified as having a medical condition: “You are correctly identified, following screening, as having a medical condition potentially dangerous for driving; you are imposed a complete cessation of driving to reduce your risk of crash.”
Driver wrongly identified as having a medical condition: “You do not have a medical condition potentially dangerous for driving, but you are wrongly identified, following screening; you are imposed a complete cessation of driving when you are actually totally able to drive.”
Driver severely injured: “You are involved in a collision and you are severely injured.”
Side effects related to self-regulation strategies: “You voluntarily decide to stop driving, because you think your ability to drive is altered or possibly altered by a medical condition; your own decision to stop driving has negative consequences on your daily living (for instance loss of autonomy, loss of mobility, loss of job...).”
Side effects after imposed cessation (following state 3): “You have been correctly identified, following screening, as having a medical condition potentially dangerous for driving; you were imposed a complete cessation of driving; this mandatory cessation of driving has negative consequences on your daily living (for instance loss of autonomy, loss of mobility, loss of job...).”
Side effects after imposed cessation (following state 4): “You have been wrongly identified as having a medical condition potentially dangerous for driving, following screening; you were imposed a complete cessation of driving, when you are actually totally able to drive; this mandatory cessation of driving has negative consequences on your daily living (for instance loss of autonomy, loss of mobility, loss of job...).”
Death: “You die as a consequence of severe crash-related consequences, or complications of consequences of driving cessation, or any other cause (age, disease...).”
Participants were instructed to use an analogical visual scale to rank their preferences from the worst (utility equals 0) to the best conceivable state (utility equals 1). An example using cancer-related states was provided, along with an instruction that participants could rank, if necessary, two states with the same preference. We then transposed measured values in utilities.
Fifty-five drivers accepted to participate (63% women); mean age of these drivers was 36 years old (SD: 9.1), most of them (90%) had a high educational level, two-thirds drove every day since an average of 16 years (SD: 8.9).
References
- 1.Brouwer W, Withaar F. Fitness to drive after traumatic brain injury. Neuropsychol Rehabilitation. 1997;7:177–93. [DOI]
- 2.Austroads Inc. Assessing Fitness to Drive for Commercial and Private Vehicle Drivers. 3rd Edition; 2003. Available at http://www.austroads.com.au/upload_files/docs/AFTD%202003-F_A-WEBREV1.pdf (accessed 21 June 2008).
- 3.Canadian Medical Association. Determining Medical Fitness to Operate Motor Vehicles. CMA Driver’s Guide, 7th Edition; 2006. Available at http://www.cma.ca/index.cfm/ci_id/18223/la_id/1.htm (accessed 21 June 2008).
- 4.Wang CC, Kosinski CJ, Schwartzberg JG, Shanklin AV. Physician’s guide to assessing and counseling older drivers. Washington, DC: National Highway Traffic Safety Administration; 2003. Available at http://www.ama-assn.org/ama/pub/category/10791.html (accessed 21 June 2008).
- 5.White S, O’Neill D. Health and relicensing policies for older drivers in the European Union. Gerontology. 2000;46:146–52. [DOI] [PubMed]
- 6.Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer’s disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54:2205–211. [DOI] [PubMed]
- 7.Dobbs BM, Carr DB, Morris JC. Evaluation and management of the driver with dementia. Neurologist. 2002;8:61–70. [DOI] [PubMed]
- 8.Fitten LJ. Driver screening for older adults. Arch Intern Med. 2003;163:2129–31. discussion 2131. [DOI] [PubMed]
- 9.Charlton J, Koppel S, O’Hare M, et al. Influence of chronic illness on crash involvement of motor vehicle drivers. Monash: Monash University Accident Research Centre; 2004:482.
- 10.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–58. [DOI] [PubMed]
- 11.Johns M. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. [DOI] [PubMed]
- 12.Maislin G, Pack A, Kribbs N. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. [DOI] [PubMed]
- 13.Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170:371–6. [DOI] [PubMed]
- 14.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–37. [DOI] [PubMed]
- 15.Retchin SM, Hillner BE. The costs and benefits of a screening program to detect dementia in older drivers. Med Decis Making. 1994;14:315–24. [DOI] [PubMed]
- 16.Viamonte SM, Ball KK, Kilgore M. A cost-benefit analysis of risk-reduction strategies targeted at older drivers. Traffic Inj Prev. 2006;7:352–9. [DOI] [PubMed]
- 17.Leproust S, Lagarde E, Salmi LR. Systematic screening for unsafe driving due to medical conditions: still debatable. BMC Public Health. 2008;8:27. [DOI] [PMC free article] [PubMed]
- 18.[Grands thémes de la sécurité routière en France]. Paris: Observatoire National Interministériel de Sécurité Routière; 2006. Available at http://www.centre.equipement.gouv.fr/IMG/pdf/1er_quad_2005_cle03aeb8.pdf (accessed 21 June 2008).
- 19.Ramaroson H, Helmer C, Barberger-Gateau P, Letenneur L, Dartigues J. Prévalence de la démence et de la maladie d’Alzheimer chez les personnes de 75 ans et plus : données réactualisées de la cohorte Paquid. Rev Neurol. 2003;159:405–11. [PubMed]
- 20.Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–9. [PubMed]
- 21.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. [DOI] [PubMed]
- 22.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–9. [DOI] [PubMed]
- 23.Ravaglia G, Forti P, Maioli F, et al. Incidence and etiology of dementia in a large elderly Italian population. Neurology. 2005;64:1525–30. [DOI] [PubMed]
- 24.Nabi H, Gueguen A, Chiron M, Lafont S, Zins M, Lagarde E. Awareness of driving while sleepy and road traffic accidents: prospective study in GAZEL cohort. BMJ. 2006;333:75. [DOI] [PMC free article] [PubMed]
- 25.Marottoli RA, de Leon CFM, Glass TA, Williams CS, Cooney LM Jr., Berkman LF. Consequences of driving cessation: decreased out-of-home activity levels. J Gerontol B Psychol Sci Soc Sci. 2000;55:S334–40. [DOI] [PubMed]
- 26.Pierce RJ. Driver sleepiness: occupational screening and the physician’s role. Aust N Z J Med. 1999;29:658–61. [DOI] [PubMed]
- 27.Bearpark H, Fell D, Grunstein R, Leeder S, Berthon-Jones M, Siullivan C. Road Safety and Pathological Sleepiness: The Role of Sleep Apnoea: Road Safety Bureau Consultants; Australian Transport Safety Bureau, Austrialia;1990.
- 28.Horstman S, Hess C, Basetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnoea patients. Sleep. 2000;23:1–7. [PubMed]
- 29.Barbe F, Pericas J, Munoz A, et al. Automobile accidents in patients with sleep apnoea syndrome: an epidemiological and mechanistic study. Am J Respir Crit Care Med. 1998;158:18–22. [DOI] [PubMed]
- 30.Taux de mortalité en 2005] : Institut National d’Études Démographiques. Available at http://www.ined.fr/fr/pop_chiffres/france/mortalite_causes_deces/taux_mortalite_sexe_age/ (accessed 21 June 2008).
- 31.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–77. [DOI] [PubMed]
- 32.Naimark D, Krahn MD, Naglie G, Redelmeier DA, Detsky AS. Primer on medical decision analysis: Part 5–Working with Markov processes. Med Decis Making. 1997;17:152–9. [DOI] [PubMed]
- 33.Hakamies BL, Johansson K, Lundberg C. Medical screening of older drivers as a traffic safety measure: A comparative Finnish-Swedish evaluation study. J Am Geriatr Soc. 1996;44:650–3. [DOI] [PubMed]
- 34.Bharucha AJ, Pandav R, Shen C, Dodge HH, Ganguli M. Predictors of nursing facility admission: a 12-year epidemiological study in the United States. J Am Geriatr Soc. 2004;52(3):434–9. [DOI] [PubMed]
- 35.Ruitenberg A, Kalmijn S, de Ridder MA, et al. Prognosis of Alzheimer’s disease: the Rotterdam Study. Neuroepidemiology. 2001;20(3):188–95. [DOI] [PubMed]
- 36.Marottoli RA, Mendes de Leon CF, Glass TA, et al. Driving cessation and increased depressive symptoms: prospective evidence from the New Haven EPESE. Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 1997;45(2):202–6. [DOI] [PubMed]
- 37.Marshall SC, Spasoff R, Nair R, van Walraven C. Restricted driver licensing for medical impairments: does it work? Can Med Assoc J. 2002;167:747–51. [PMC free article] [PubMed]
- 38.Owsley C, McGwin G Jr., Phillips JM, McNeal SF, Stalvey BT. Impact of an educational program on the safety of high-risk, visually impaired, older drivers. Am J Prev Med. 2004;26:222–9. [DOI] [PubMed]
- 39.Orth M, Duchna HW, Leidag M, et al. Driving simulator and neuropsychological testing in OSAS before and under CPAP therapy. Eur Respir J. 2005;26:898–903. [DOI] [PubMed]
- 40.Frey JG. Syndrome des apnées obstructives du sommeil et accidents automobiles. Rev Med Suisse. 2005;23:1561–2. 1564. [PubMed]
- 41.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes (Third Edition). Oxford University Press; 2005.
- 42.Adler G, Rottunda S, Dysken M. The older driver with dementia: an updated literature review. J Safety Res. 2005;36(4):399–407. [DOI] [PubMed]
- 43.Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–7. [DOI] [PMC free article] [PubMed]
- 44.Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry 2008 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 45.Leproust S, Lagarde E, Suissa S, Salmi LR. Association between road vehicle collisions and recent medical contact in older drivers: a case-crossover study. Inj Prev. 2007;13(6):382–7. [DOI] [PMC free article] [PubMed]


