Figure 5. c-MYC is mediator of GSK3 inhibition-mediated cytotoxicity.
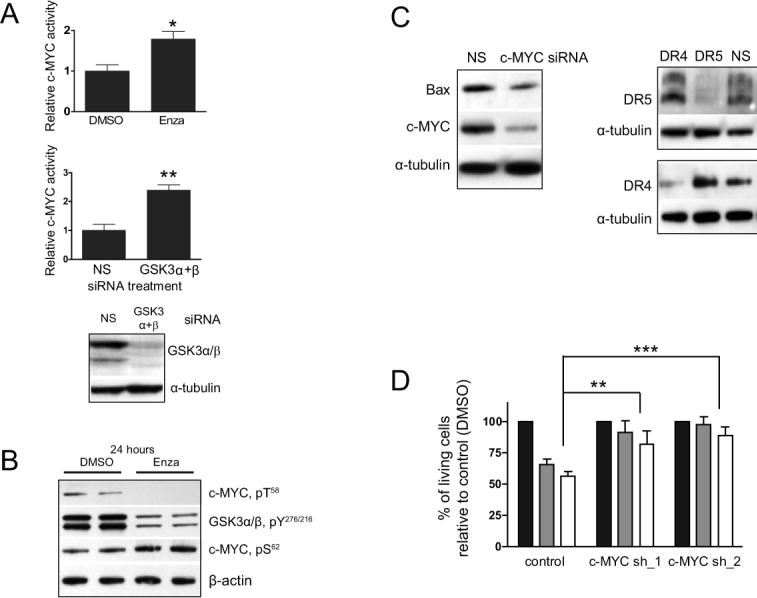
(A) GSK3 inhibition by siRNA and enzastaurin up-regulates c-MYC activity as revealed by ELISA-based DNA-binding assay (B) WB analysis of nuclear extracts prepared from cells treated with 5 μM enzastaurin or DMSO. c-MYC phosphorylation at T58 is down-regulated in U251. This correlates with reduced Y phosphorylation of GSK3, both at 4 and 24 hours (shown) of treatment with enzastaurin. Conversely, S62 phosphorylation of c-MYC was up-regulated at 24 hours (C) Western Blot of total cell lysates: c-MYC siRNA negatively regulates Bax, DR4 and DR5 proteins in U251. NS, non-silencing control siRNA. (D) Silencing of c-MYC in U251 by shRNA in two individual clones, c-MYC-shRNA_1 & 2, effectively protects cells from 0.5 μM 705701 or from 0.5 μM LY2064827 (24 hours treatment) compared to control cells. Cells were plated at a density of 50,000 per well in a 6-well plate.
