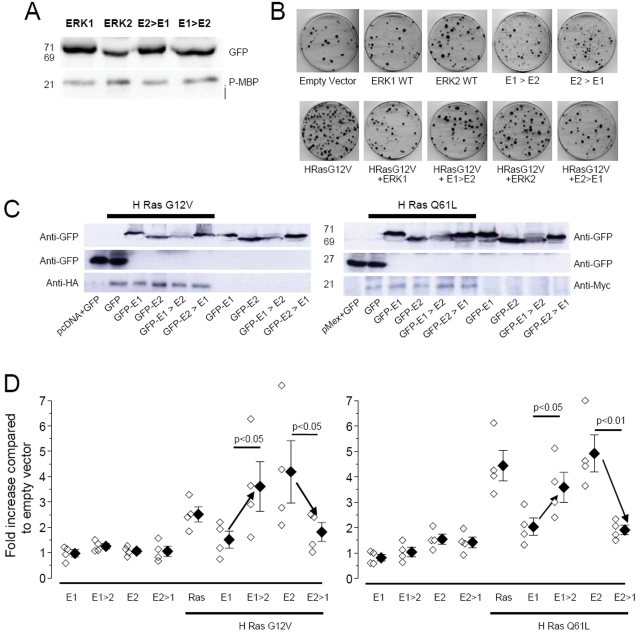
Figure 8. Functional consequences of N-terminus mutations of ERK1 and ERK2.
A) In vitro assay testing the capacity of the indicated ERK to be activated by MEK and to phosphorylate the downstream target MBP. The upper lanes show the purified protein revealed by the anti-GFP antibody. The fusion proteins showed correct molecular weights (ERK1, E2>E1 71 kD; ERK2, E1>E2 69 kD). The lower panel indicates that each protein is able to phosphorylate MBP. The experiment was performed in triplicate with similar results. B) Representative examples of the cell colonies transfected with the indicated constructs. C) Immunoblot anti GFP shows that all the colonies expressed the GFP tagged proteins; the anti-HA and anti-Myc staining show that the mutated forms of Ras (respectively: H-Ras G12V and H-Ras Q61L), were expressed in the colonies with constitutive activation of the pathway, but they were absent in the wild type background. D) Quantification of the effect on proliferation of the various expressed fusion proteins. The symbols indicates the number of colonies counted after transfection with the specified vector, normalized to the colonies counted after transfection with the empty vector. The empty symbols represent the result of a single experiment and the filled symbols are the averages of each group. Expression of constitutively active Ras (H-Ras G12V and H-Ras Q61L) causes a large increase in proliferation that is inhibited by co-expressing ERK1 but not by co-expressing ERK2. The mutant of ERK1 characterized by fast shuttling (E1Δ39 indicated as E1>E2) did not prevent H-Ras G12V and Q61L-induced proliferation behaving similarly to ERK2. In contrast the slow mutant of ERK2 (Δ39E2 indicated as E2>E1) inhibited proliferation. Statistical significativity was essayed by t-test.