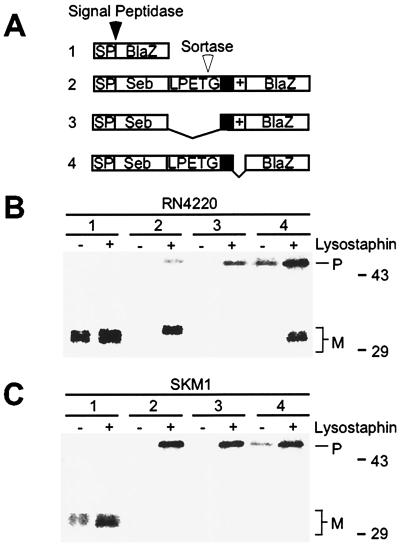
Figure 3.
Protein secretion and sorting pathways of staphylococci. (A) Drawing depicts the structure of protein fusions with the mature domain of staphylococcal β-lactamase (BlaZ). 1, (SebSP-BlaZ) fusion of the enterotoxin B signal peptide (SP). 2, (Seb-Cws-BlaZ) fusion of enterotoxin B (Seb) and the protein A sorting signal to BlaZ. 3, (Seb-CwsΔLPXTG-BlaZ) same fusion as in 2 but lacking the LPXTG motif. 4, (Seb-CwsΔR-BlaZ) same fusion as in 2 but lacking the retention signal (+). (B) Pulse-labeled staphylococcal cultures (strain RN4220) were divided into two aliquots and precipitated with TCA. One sample was directly boiled in SDS, whereas the other was first subjected to peptidoglycan hydrolysis with lysostaphin and then boiled in SDS. Samples were subjected to immunoprecipitation with anti-BlaZ (α-BlaZ) and analyzed by SDS/PAGE and PhosphorImager. (C) Same experiment as in B, but using the sortase mutant strain SKM1.