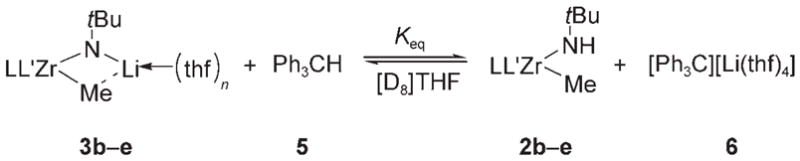
Table 1.
Acidity of methyl amide complexes 2 b–e.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | Complex | Ligand | Yield [%][b] | Keq[c] | (pKa)THF |
| 1 | 2 b | Cp*2 | 33 | 0.249 ± 0.007 | 29.8 |
| 2 | 2 c | Cp*CpMe4 | 42 | 0.524 ± 0.010 | 30.1 |
| 3 | 2 d | CpMe42 | 51 | 1.05± 0.03 | 30.4 |
| 4 | 2 e | rac-(ebthi) | 22 | 0.079 ± 0.005 | 29.3 |
Reactions were run with 0.3 equiv [12]crown-4.
Yield determined by NMR spectroscopy and calculated using 1,3-dimethoxy-5-methylbenzene as an external standard.
Values are reported as the average of three runs ± standard deviation.
