Table 2.
The coupling reaction of pyrrol– and indol–substituted aniline and terminal alkynes.a
| entry | amine | alkyne | product | 4 (mol %) | t (h) | yd (%)b |
|---|---|---|---|---|---|---|
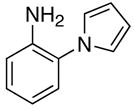
|
|
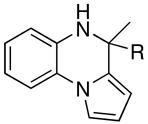
|
||||
| 1 | R = Et | 7a | 1 | 24 | 96 | |
| 2 | R = Ph | 7b | 2 | 24 | 93 | |
| 3 | R = C6H4-p-OMe | 7c | 2 | 20 | 90 | |
| 4 | R = 2-SC4H3 | 7d | 5 | 24 | 92 | |
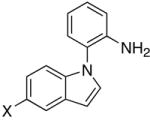
|
|
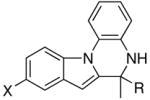
|
||||
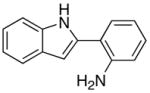
| 5 | X = H | R = Me | 8a | 2 | 20 | 93 |
| 6 | X = H | R = p-Tol | 8b | 3 | 24 | 75 |
| 7 | X = H | R = C6H4-p-Cl | 8c | 3 | 24 | 70 |
| 8 | X = OMe | R = Et | 8d | 3 | 24 | 82 |
|
|
|
||||
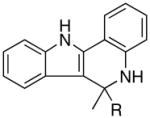
| 9 | R = n-Bu | 9a | 5 | 20 | 70 | |
| 10 | R = Ph | 9b | 5 | 24 | 91 |
Reaction conditions: amine (1.5 mmol), alkyne (2–5 mmol), Ru3(CO)12/HBF4·OEt2 (1:3), benzene (2–5 mL), 90–95 °C.
Isolated yield based on amine.
