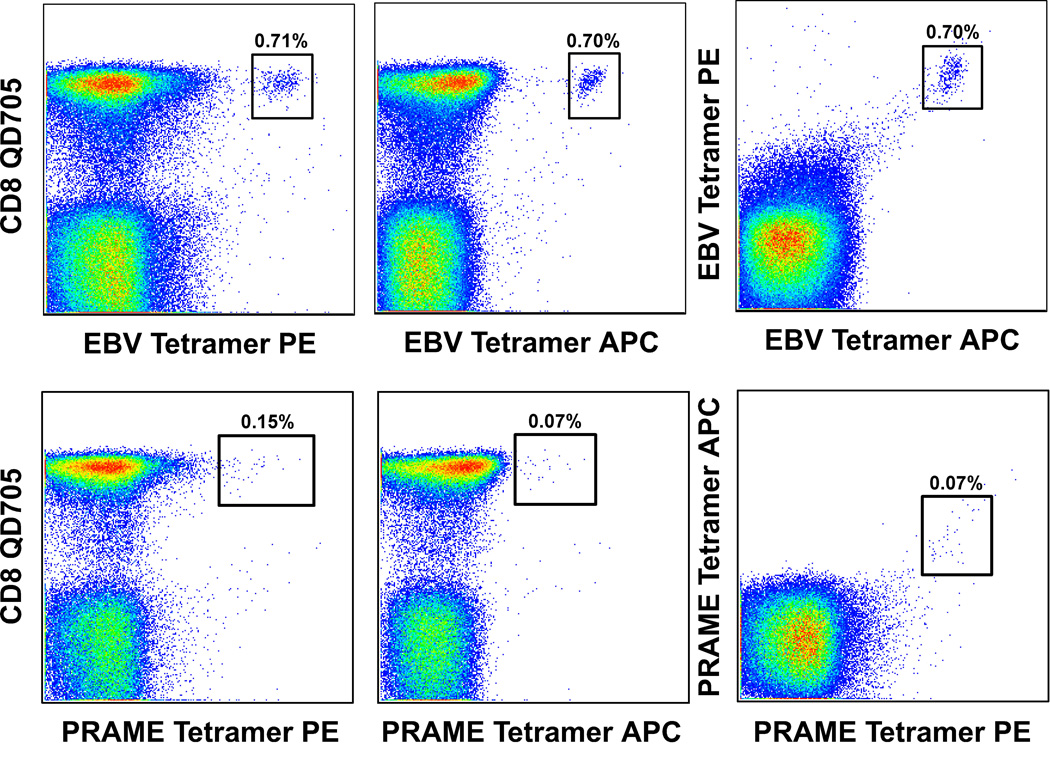
Figure 3. Discrimination of true tetramer binding events by double fluorochrome verification.
PBMC were stained with BiViD or ViViD, pre-titered Epstein-Barr virus (EBV) BMLF1259–267/HLA A*0201 (top row) or preferentially expressed antigen on melanomas (PRAME)100–108/HLA A*0201 (bottom row) tetramers and mAbs specific for cell surface markers as described in the legend to Figure 2. Antigen-specific CD8+ T cells identified with phycoerythrin (PE)-conjugated or APC-conjugated pHLA A*0201 tetramers are shown in the left and middle panels respectively. As shown, background staining can be more pronounced with PE-conjugated pMHCI tetramers due, in part, to greater spreading error and the tendency for heterogeneity within such preparations (17,20). Random background originating from individual tetramers representing a given antigen specificity can be discerned by staining with a mixture of differentially conjugated tetramers together (right panel). Cognate CD8+ T cells will capture pMHCI tetramers from solution regardless of the associated fluorochrome; singly stained cells likely represent aberrant binding events. In these experiments, pre-mixing of tetramer solutions prior to staining minimizes the bias that can arise from sequential additions due to rapid on-rates for binding (26). Nevertheless, it remains possible that some cognate CD8+ T cells will stain with only one of the corresponding pMHCI tetramers simply by chance, although this is unlikely given that many fluorochromes must be taken up for an individual cell to be visible by flow cytometry. Furthermore, doubly stained cognate CD8+ T cell populations exhibit reduced individual fluorescence intensities due to competition between tetramers for a finite number of surface TCRs. Thus, this technique is best considered as confirmatory for situations in which low frequency antigen-specific CD8+ T cells are difficult to discriminate reliably; this is especially pertinent when traditional gating trees are used (Figure 2).