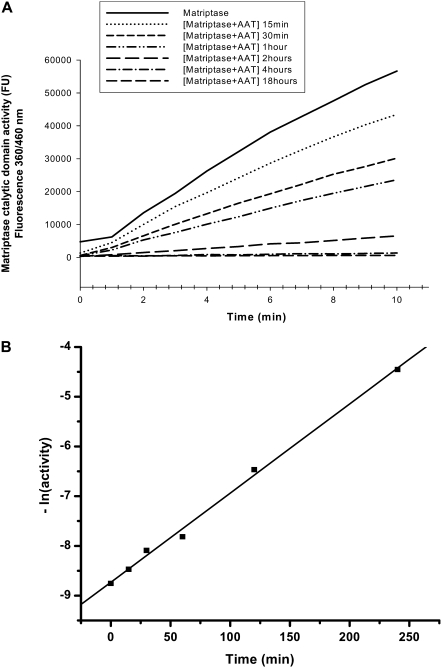
Figure 8.
Determination of reaction rate constant (k). (A) Kinetic curves of matriptase activity (cleavage of substrate) measured after pre-incubating matriptase catalytic domain (5 nM) with vehicle or with native AAT (960 nM) for 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, and 18 hours. Three independent experiments were performed, with five repeats each. Figure shows one representative experiment of five repeats. (B) As described in Materials and Methods, the matriptase activity was calculated from A, and the exponential free enzyme activity was plotted versus time of incubation with AAT. A good linear fit was obtained (R = 0.99812). The k was calculated as 0.31 × 103 M−1s−1 from the slope of the plot (kobs = k × [AAT]0 = 0.01794 min−1). This experiment was repeated twice, with identical results.