Abstract
Aim
Excessive oxidative stress has been implicated in the pathology and complications of diabetes, which leads to myocardial ischemia reperfusion injury. The present study was designed to examine whether resveratrol (trans-3,5,4′-trihydroxystilbene), a polyphenolic compound present in red wine has a direct cardioprotective effect on diabetic myocardium.
Methods
Resveratrol (2.5mg/kg b.wt/day) and L-NAME (25mg/kg b.wt/day) were administered orally for 15 days to streptozotocin (65mg/kg) induced diabetic rats. Sprague Dawley rats were divided into 5 groups i) Control ii) Diabetic iii) Diabetic+resveratrol iv) Diabetic+Resveratrol+L-NAME (nitric oxide synthase inhibitor), v) Diabetic+L-NAME. In our present study resveratrol demonstrated significant reduction in glucose level in diabetic rats. After the treatment, the hearts were excised and subjected to 30 min of global ischemia followed by 2 hours of reperfusion.
Results
Resveratrol treated diabetic rats demonstrated significant reduction in glucose levels as compared to the non treated diabetic animals, improved left ventricular function throughout reperfusion compared to the diabetic or L-NAME treated animals (dp/dtmax 1457 ± 51 vs 999 ± 44 mmHg/sec at 120 min reperfusion). Cardioprotection from ischemic injury in resveratrol treated diabetic rats showed decreased infarct size (42% vs 51%) and cardiomyocyte apoptosis (35% vs 40%) by TUNEL assay. Resveratrol produced significant induction of p-AKT, p-eNOS, Trx-1, HO-1 and VEGF in addition with increased activation of Mn-SOD activity in diabetic animals compared to non-diabetic animals. However treatment with L-NAME in resveratrol treated and non-treated diabetic animals demonstrated significant downregulation of the above mentioned protein expression profile and MnSOD activity.
Conclusion
In the present study we found that the mechanism (s) responsible for the cardioprotective effect of resveratrol in the diabetic myocardium include upregulation of Trx-1, NO/HO-1 and VEGF in addition with increased MnSOD activity and reduced blood glucose level. Thus this study shows a novel mechanism of pharmacological preconditioning with resveratrol in the diabetic myocardium.
Keywords: Diabetes, ischemia reperfusion, Thioredoxin, Mn-SOD, HO-1, Glucose
INTRODUCTION
Coronary artery disease leading to myocardial infarction and heart failure is a chronic complication of diabetes (1, 2). After a myocardial ischemic event, diabetes is associated with increased adverse outcomes in terms of both morbidity and mortality over the short and long term (3). The mortality rate in the diabetic patients after myocardial infarction is twice that of non-diabetic patients (4). Capillary density and diameter also exhibit a progressive reduction of more than 20% over 26 week of diabetes (5).
Kondo and Kahn (6) reported that different defects in components of cell survival kinase cascades in diabetic models are not species specific but organ specific within the same species. In both Type I and Type II diabetic mice, they showed that in the retina, the reduction in the phosphorylation of PDK1 and AKT was due to reduction of total levels of PDK1 and AKT when compared with controls after insulin stimulation. Hyperglycemia has been shown to inhibit the prosurvival effect of VEGF, leading to retinal cell apoptosis via tyrosine nitration of PI3Kinase that results in Akt inactivation and increased p38 mitogen-activated protein kinase activation (7).
Resveratrol (trans-3,5,4′-trihydroxystilbene), a polyphenolic compound and naturally occurring phytoalexin, has been designated the active agent (8) present in red wine. In our previous study both red wine extract and resveratrol reduced the amount of oxidative stress and upregulated inducible nitric oxide synthase (iNOS) mRNA expression leading to reduction of cardiomyocyte apoptosis and infarct size. One of our recent study showed angiogenic properties of resveratrol through the induction of VEGF, iNOS and eNOS expression, which provided significant cardioprotection as evidenced by the reduction of infarct size and increased capillary density (9) in the rat myocardial infarction model.
In the present study, we demonstrated how diabetes mediated abnormalities can be reversed by resveratrol treatment and marked changes in the myocardium can be prevented by early resveratrol treatment through nitric oxide dependent mechanisms. In our study, we document that resveratrol possess significant hypoglycemic effect in STZ-induced diabetic rats over a 30 day experimental period. The definite mechanism for the beneficial effects of resveratrol may be due to nitric oxide (NO) production in endothelial cells (10), in the kidney (11), and in the heart (12). Giovannini et al (11) and Naderali et al (13) demonstrated that upregulation of NO is a principal factor for the anti-ischemic function of resveratrol. One of our previous study, also clearly documented that the anti-ischemic effects of resveratrol was blocked by NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO synthesis, indicating that NO is the mediator of resveratrol preconditioning of the heart (12). Another interesting study revealed, a strong reciprocal relationship between VEGF and NO even in a rat model with chronic NO blockade (14). Most of the protective biological actions associated with resveratrol was found to be associated with its intrinsic radical scavenging properties. Hyperglycemia in diabetes leads to cardiovascular complications induced by oxidative stress (15). It is, therefore, anticipated that modulation of redox signaling may be causally related to cardioprotection mediated by resveratrol. Concomitant with this hypothesis, in our previous study resveratrol-treated myocardium after MI significantly induced thioredoxin (Trx-1), heme oxygenase (HO-1) and VEGF expression along with increased capillary density. This resveratrol-mediated cardioprotection and angiogenic response via upregulation of VEGF was blocked by SnPP (HO-1 inhibitor) (9,15).
Thioredoxins are the major cellular protein disulfide reductases ubiquitously present in mammalian tissues including heart. They possess dithiol/disulfide active site and can serve as electron donors for enzymes including thioredoxin peroxidases and ribonucleotide reductases (16) Thioredoxins are critical for redox regulation of protein function and signaling via thiol-redox control. Thioredoxins are reduced by electrons from NADPH via thioredoxin reductase. HO-1 is expressed ubiquitously in many cell types, and its transcription is activated by numerous pro-oxidant molecules, including haem, heavy metals, oxidized LDL (low-density lipoprotein), proinflammatory cytokines and ROS (17–19). HO-1 catalyzes heme to carbon monoxide (CO) and biliverdin which is further reduced to bilirubin (20). Anti-inflammatory, anti-apoptotic properties of CO (21) and antioxidant property of bilirubin has been reported (22). Moreover delivery of adeno-associated virus HO-1 gene delivery resulted in acute myocardial protection in a rat model of acute ischemia-reperfusion injury (23).
Looking at the tremendous potential of resveratrol in cardioprotection, we used this polyphenol to study its beneficial effect on diabetic ischemic reperfused myocardium. As expected resveratrol demonstrated reduction in glucose level in diabetic rats. Resveratrol treated diabetic rats demonstrated improved left ventricular function throughout reperfusion compared to the diabetic or L-NAME treated animals. Western blot revealed induction of p-Akt, Trx-1 and HO-1 in the treatment group. We also observed an increase in antioxidative enzyme, MnSOD activity along with decreased cardiomyocyte apoptosis in resveratrol treated myocardium. Taken together, these results demonstrated resveratrol as a potent pharmacological agent in reducing diabetic induced ischemia reperfusion injury.
MATERIALS AND METHODS
Experimental animals
This study was performed in accordance with the principles of laboratory animal care formulated by the National Society for Medical Research and with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication No. 85–23, revised 1985). The experimental protocol was approved by the Institutional Animal Care Committee of the Connecticut Health Center (Farmington, CT). Male SD rats (250–275gm) were randomly separated into normal and diabetic rats as they received vehicle (0.1mol/l citrate buffer, pH 4.5) alone or streptozotocin (STZ; Sigma, St Louis, MO) (pH 4.5) at a dosage of 65mg/kg body weight. Five days after STZ injection, hyperglycemia was documented by measuring the glucose content of tail vein blood with blood glucose monitoring system (Thera Sense, Inc. Alameda, CA, USA). Rats with blood glucose concentrations ≥300mg/dl were considered to be diabetic.
Experimental protocol
Rats were randomly divided into 5 groups: 1) Non diabetic rats (control); 2) STZ hyperglycemic rats (Dia); 3) diabetic STZ rats treated with Resveratrol (Dia + Rsvl) (2.5mg/kg body weight; Sigma, St. Louis, MO); 4) diabetic STZ rats treated with Resveratrol (2.5mg/kg body weight) and N(G)-nitro-L-arginine methyl ester (L-NAME, Sigma, St. Louis, MO), a nitric oxide synthase inhibitor (25mg/kg body weight) (Dia + Rsvl + L-NAME); 5) diabetic STZ rats treated L-NAME (Dia + L-NAME). Resveratrol and L-NAME was orally administered to rats for 15 consecutive days after 15 days of STZ injection. After the treatment period, the rats were fasted overnight, and the hearts were removed and subjected to 30 minutes of ischemia followed by two hours of reperfusion.
Isolated working heart prepration
The procedure for creating isolated perfused rat hearts was similar to that previously described (24). The Langendorff preparation was switched to the working mode following the washout period as previously described (25). At the end of 10 min, after the attainment of steady state cardiac baseline functional parameters were recorded. The circuit was then switched back to the retrograde mode and hearts were perfused for 5 min with KHB buffer, and then the hearts were subjected to 30 minutes of global ischemia followed by 2 h of reperfusion. Aortic pressure was measured using a pressure transducer (Micro-Med, Inc.,) and the signal was amplified using a HPA-400 (Micro-Med, Inc, USA. Heart Rate (HR), left ventricular developed pressure (LVDP), and (dP/dtmax) were all derived or calculated from the continuously obtained pressure signal (26). Aortic flow (AF) was measured using a calibrated flow meter (Gilmont Instrument Inc., Barrington, IL, USA) and coronary flow (CF) was measured by timed collection of the coronary effluent dripping from the heart (25).
Infarct size estimation
At the end of reperfusion, a 1% (w/v) solution of triphenyl tetrazolium chloride in phosphate buffer was infused into the aortic cannula for 1 min at 37 °C. To quantify the areas of interest in pixels, Scion image analysis software was used. The infarct size was quantified and expressed in pixels (27) (n=6 in each group).
Determination of cardiomyocyte apoptosis
Terminal dUTP nick end labeling (TUNEL) assay was performed in deparafinized sections (n=6 in each group) of 4uM thick with an Apop Tag Kit (Oncor Inc) (28, 29)
Quantitative real-time RT-PCR
Reverse transcription reaction (RT) was performed with 1 μg total RNA isolated from left ventricular tissue of baseline samples (perfused for 3–5mins to remove the blood) of all the groups (n=6 in each group) (28). Real-time RT-PCR analysis was carried out with 10ng of RT product by iCycler iQ detection system (Biorad, Hercules, CA) using Syber Green I fluorescence and β-actin as reference control. The primer sequences used for real-time RT-PCR for HO-1, forward, 5′-AAGAGGCTAAGACCGCCTTC-3′; reverse, 5′-CCTCTGGCGAAGAAACTCTG -3′;VEGF, forward, 5′ TGTGCGGGC TGCTGCAATGAT-3′; reverse, 5′-TGTGCTGGCTTTGGTGAGGTTTGA -3′; and TRX-1, forward, 5′-TCCAATGTGGTGTTCCTTGA -3′; reverse, 5′-ACCAGA GAACTCCCCAA CCT -3′respectively.
Western blot analysis
We prepared cytosolic or nuclear protein from the left ventricular tissue either from baseline (3–5 mins of perfusion to remove the blood) or 120 min after reperfusion (n=6 in each group) using the commercially available kit (Sigma, Saint Louis, MO). Standard Western Blot analysis was performed using antibodies to Ser473-phospho-Akt, Akt, Ser1177-p-eNOS, eNOS (Cell Signaling, Danvers, MA), VEGF (R&D Systems, Inc., Minneapolis, MN, USA), HO-1(Stressgen Bioreagents, Ann Arbor, Michigan, USA) and Trx-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Primary antibody binding was visualized by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (14).
Manganese Superoxide Dismutase (MnSOD) assay
A commercially available superoxide dismutase assay kit (Calbiochem, San Diego, CA, USA) was used according to the manufacturer’s instructions to selectively determine the activity of the mitochondrial isoform, MnSOD (n=6).
Statistical analysis
Results are expressed as mean ± standard error of the mean (± S.E.M.). ANOVA with the Bonferroni post hoc test was used to test for differences, and values were considered to be significant at P < 0.05.
RESULTS
Effect of resveratrol on the blood glucose
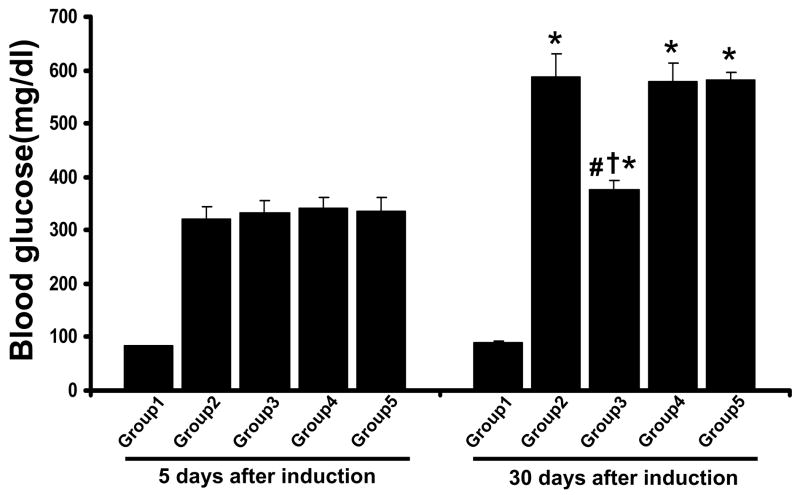
Blood glucose levels were measured after 5 days of STZ treatment and compared to control animals to confirm the increased blood glucose levels. Rats with blood glucose concentrations ≥300mg/dl were considered to be diabetic. Following 15 days of STZ injection resveratrol and L-NAME were administered orally as mentioned above. The blood glucose levels were measured in each animal (n=12 in each group) to determine the extent of hyperglycemia. Blood glucose levels were significantly increased in the diabetic rats (>500mg/dl) when compared to non-diabetic rats (90mg/dl). Treatment with resveratrol decreased the blood glucose level significantly in Dia + Rsvl group (370mg/dl) when compared to diabetic group. L-NAME treated groups have abolished the resveratrol mediated reduction in blood glucose level (Figure 1).
Figure 1. Effect of resveratrol on blood glucose.
Results are expressed as mean ± SEM. (n=6) *p<0.05 groups 2, 3, 4 & 5 vs group 1; †p<0.05 groups 3, 4 & 5 vs group 2; #p<0.05 groups 3 vs group 4 & 5. Where Group 1-control rats, Group 2 -diabetic rats, Group 3 -diabetic rats treated with resveratrol, Group 4 -diabetic rats treated with resveratrol + L-Name, Group 5-diabetic rats treated with L-Name.
Effect of resveratrol on myocardial function
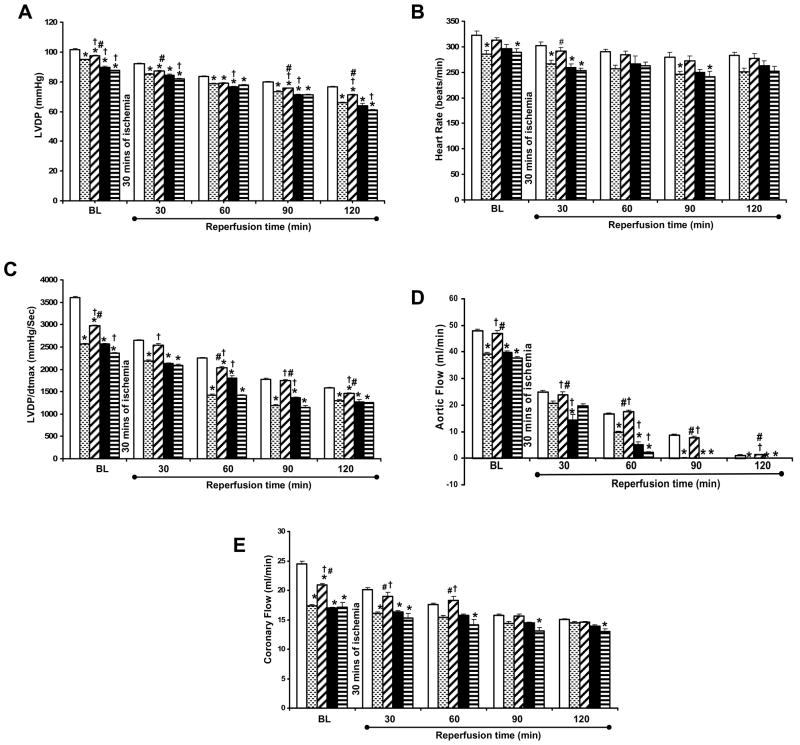
There was a significant difference in the functions at the baseline level in between the groups. The left ventricular developed pressure (Figure 2A) was found to be significantly decreased in the Dia group (94.83 ± 0.30) as compared to the non-diabetic control (101.72 ± 0.50). Rsvl treatment was found to improve LVDP in Dia + Rsvl (97.66 ± 0.30) as compared to the diabetic group. LVDP after reperfusion (2 hrs) was significantly decreased in the diabetic group (66.75 ± 0.79) and resveratrol treatment in Dia + Rsvl group (71.15±0.34) showed a significant increase in the LVDP as compared to the diabetic group. Decreased LVDP was observed in L-NAME treated groups Dia + Rsvl + L-NAME (63.96 ± 1.48), and Dia + L-NAME group (60.83 ± 0.60).
Figure 2. A–E. Effect of resveratrol on LVDP (A), Heart rate (B), dp/dtmax (C), Aortic flow (D) and Coronary flow (E) in STZ induced diabetic rats.
The isolated hearts from rats of all the five groups (n=6) were subjected to 30 mins of global ischemia followed by 2 hrs of reperfusion. Results are expressed as mean ± SEM. *p<0.05 groups 2, 3, 4 & 5 vs group 1; †p<0.05 groups 3, 4 & 5 vs group 2; #p<0.05 groups 4 & 5 vs group 3. Where plain bar represents group 1 (control rats), dotted bar represents group 2 (diabetic rats), wide upward diagonal bar represents group 3 (diabetic rats treated with resveratrol), black bar represents group 4 (diabetic rats treated with resveratrol + L-Name), dark horizontal bar represents group 5 (diabetic rats treated with L-Name).
The dp/dtmax at the baseline was found to be significantly decreased in the diabetic group (2561.33 ± 19.14) as compared to the non-diabetic control (3597.33 ± 34.09). Diabetic rats treated with resveratrol (Figure 2C) have shown increased dp/dtmax (2971.66 ± 20.79) as compared to diabetic group. After 2hrs of reperfusion dp/dtmax was significantly decreased in the diabetic group (1300.43 ± 18.25). Dia + Rsvl group (1457 ± 21) showed significant increase in the dp/dtmax as compared to the diabetic group. L-NAME treatment has shown decreased functional recovery in Dia + Rsvl + L-NAME (1275 ± 44.94) and Dia + L-NAME groups (1256 ± 5.60) as compared to resveratrol treated group.
The aortic flow (Figure 2D) at the baseline was found to be significantly decreased in the diabetic group (39 ± 0.49) as compared to the non-diabetic control (43.50 ± 0.62). Resveratrol treatment was found to significantly improve aortic flow in the Dia + Rsvl group (42.30 ± 1.11) as compared to the diabetic group. Aortic flow after reperfusion (2 hrs) was zero in the diabetic, Dia + Rsvl + L-NAME and Dia + L-NAME groups. Dia + Rsvl group (1.30 ± 0.16) showed a significant increase in the aortic flow as compared to the other groups. Heart rate and coronary flow was also found to have significantly improved in the Dia + Rsvl group as compared to the diabetic control both at baseline and at the end of reperfusion (Figure 2B & 2E). In all the functional parameters L-NAME treated groups has abolished the resveratrol mediated protection supporting the hypothesis that the resveratrol mediated cardioprotection is nitric oxide mediated.
Effect of Resveratrol on Myocardial Infarct Size
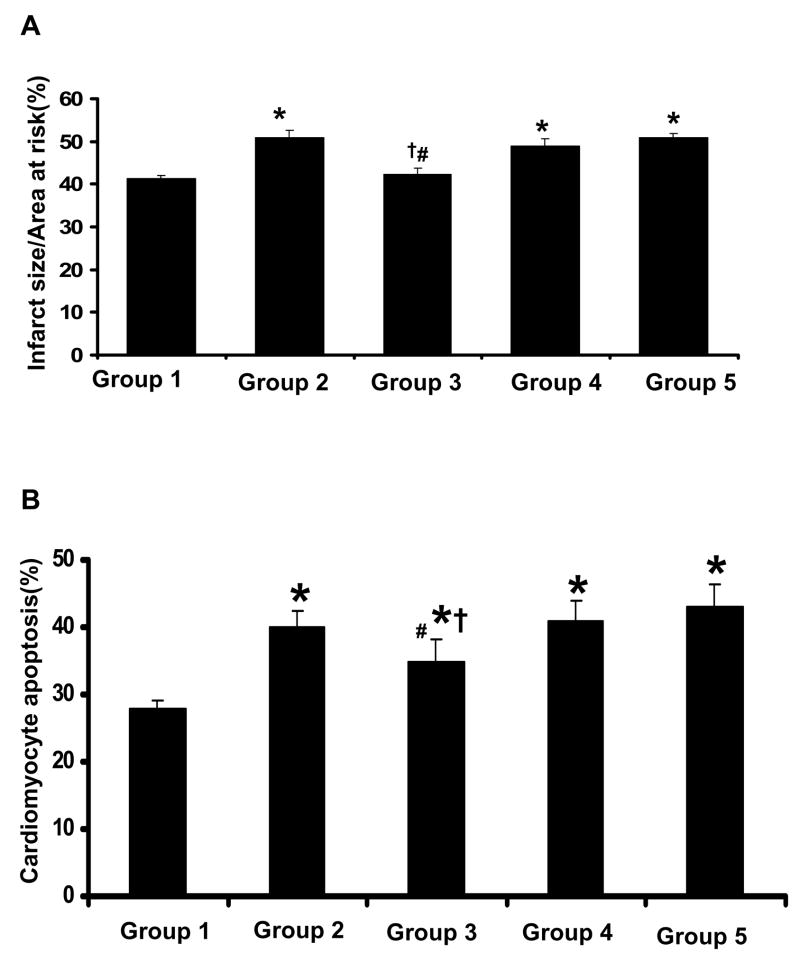
To examine whether resveratrol treatment reduces the cardiac tissue damage following I/R in diabetic myocardium myocardial infarct size was measured. Figure 3A shows the infarct size expressed as percent of infarction to total area at risk which was found to be noticeably increased to 51% in diabetic control as compared to non-diabetic control (41%). The infarct size was found to be significantly decreased in the Dia + Rsvl group (42%) group as compared to all other treated groups where as we found similar pattern (no significant difference) between resveratrol and non-diabetic control. L-NAME treated groups have shown 47% in Dia + Rsvl + L-NAME group & 49% in Dia +L-NAME group.
Figure 3. A. Effect of resveratrol on Infarct size. B: Effect of resveratrol on Cardiomyocyte Apoptosis.
The isolated hearts from rats of all the five groups (n=6) were subjected to 30 mins of global ischemia followed by 2 hrs of reperfusion. Results are expressed as mean ± SEM. *p<0.05 groups 2, 3, 4 & 5 vs group 1; †p<0.05 groups 3, 4 & 5 vs group 2; #p<0.05 groups 4&5 vs group 3. Where Group 1-control rats, Group 2-diabetic rats, Group 3-diabetic rats treated with resveratrol, Group 4-diabetic rats treated with resveratrol + L-Name, Group 5-diabetic rats treated with L-Name.
Effect of resveratrol on cardiomyocyte apoptosis by TUNEL assay
Double antibody staining with α-sarcomeric actin (Figure 3B) by TUNEL assay was used to measure the cardiomyocyte apoptosis. Diabetic myocardium has shown increased apoptotic cardiomyocytes 40% as compared to 28% in non-diabetic myocardium. Treatment with resveratrol has shown a significant decrease in the cardiomyocyte apoptosis to 35% from 40% as shown in the diabetic myocardium. The decreased cardiomyocyte apoptosis in resveratrol treated group might be due to increased expression of phosphorylated Akt and eNOS levels. L-NAME treated groups Dia + Rsvl + L-NAME and Dia + L-NAME have shown 41% and 43% respectively.
Effect of Resveratrol on the mRNA expression of Trx-1, HO-1 & VEGF by Real-time RT- PCR
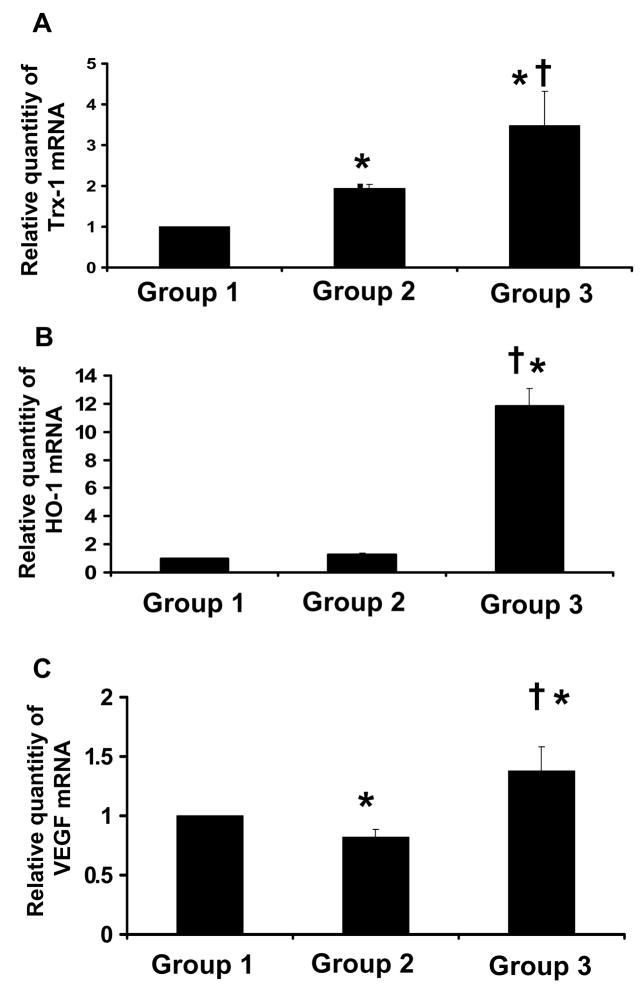
The mRNA expression of Trx-1 was increased in the resveratrol as well as in diabetic control group as compared to non-diabetic control (Figure 4A). However the increase in resveratrol treated group was 3.4 fold and in the diabetic it was 1.9 fold. Similarly HO-1 mRNA expression was significantly increased in the resveratrol treated (12 fold) and in diabetic control group (1.3) as compared to non-diabetic control (Figure 4B). The increased expression of HO-1 mRNA was observed in diabetic group however the increase was not statistically significant as compared to control. Again, the increased level of Trx-1 and HO-1 mRNA expression might be a compensatory mechanism against diabetes induced oxidative stress. In contrast VEGF mRNA expression was found to be reduced in the diabetic group to 0.82 fold when compared to non-diabetic control group. On treatment with resveratrol the expression was significantly increased to 1.37 fold in the Dia + Rsvl group (Figure 4C).
Figure 4. A–C. Effect of resveratrol on Trx-1(A), HO-1 (B) & VEGF (C) mRNA expression.
Bar graph showing relative abundance (arbitrary units) of Trx-1, HO-1 & VEGF mRNA at the baseline level by quantitative real-time RT-PCR analysis (n=6). *p<0.05 Groups 2&3 vs Group 1; †p<0.05 Groups 3 vs Group 2; Where Group 1-control rats, Group 2-diabetic rats, Group 3-diabetic rats treated with resveratrol.
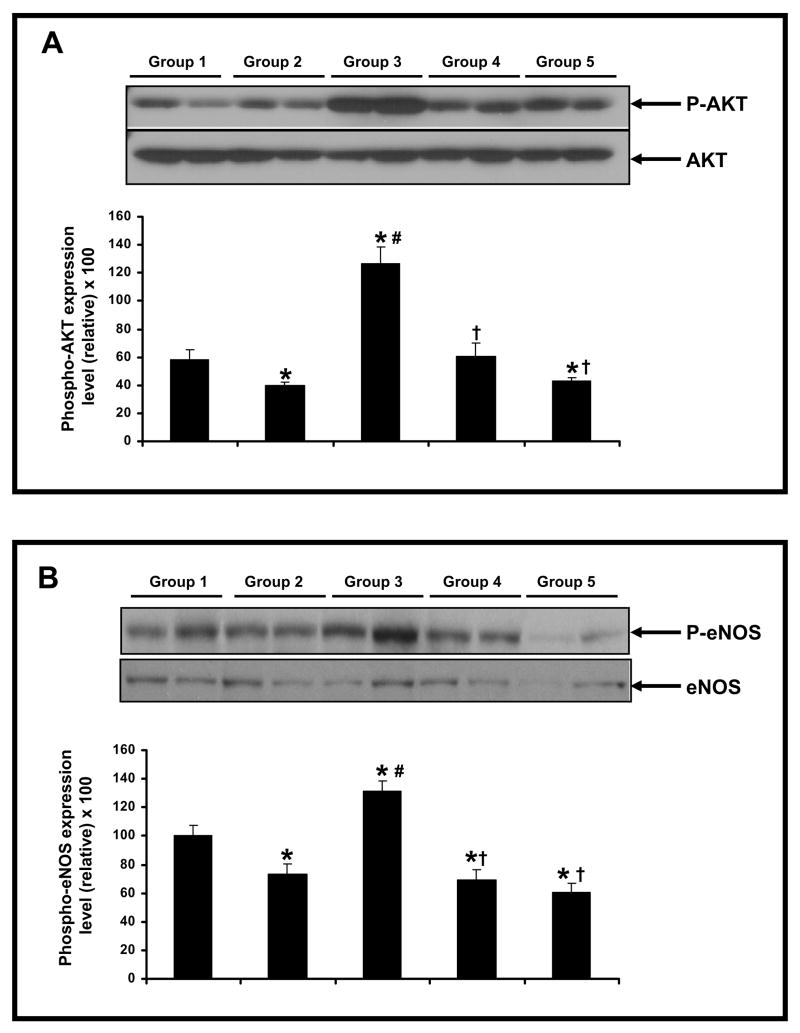
Effect of Resveratrol on the protein phosphorylation of Akt & eNOS
The p-Akt level was found to be increased following 30 minutes of ischemia and 2 hours of reperfusion (Figure 5A) in resveratrol treated group when compared to the diabetic and non-diabetic control group. However, no statistical difference was found between diabetic and non-diabetic control groups. L-NAME treated groups (Dia + Rsvl + L-NAME group & Dia + L-NAME group) have shown decreased phosphorylation of Akt when compared to Dia + Rsvl group. The resveratrol mediated upregulation of p-Akt level was abolished in L-NAME treated groups (Figure 5A). Similarly, the p-eNOS level (Figure 5B) following 30 minutes of ischemia and 2 hours of reperfusion was found to be increased in resveratrol treated group when compared to diabetic and L-NAME treated groups (Dia + Rsvl + L-NAME group & Dia + L-NAME group). We found no statistical difference in diabetic and non-diabetic control groups. Akt and eNOS were used as the loading controls for phosphorylated/active forms of Akt and eNOS. The increased phosphorylation of Akt and eNOS levels following ischemia/reperfusion in resveratrol (Dia + Rsvl) treated group might be due do increased non-phosphorylated levels of these proteins to compensate the stress induced in the diabetic myocardium resulting in reduced cardiomyocyte apoptosis and myocardial infarct size.
Figure 5.
A–B. Representative Western blot showing the effect of resveratrol on the phosphorylation of AKT (A) & eNOS (B) in streptozotocin induced diabetic ischemic reperfused rat hearts. The hearts (n=6) from each group were isolated and subjected to 30 mins of global ischemia followed by 2 hrs of reperfusion. *p<0.05 groups 2, 3, 4 & 5 vs group 1; †p<0.05 groups 3, 4 & 5 vs group 2; #p<0.05 groups 3 vs group 4 & 5. Where Group 1-control rats, Group 2-diabetic rats, Group 3-diabetic rats treated with resveratrol, Group 4-diabetic rats treated with resveratrol + L-Name, Group 5 -diabetic rats treated with L-Name.
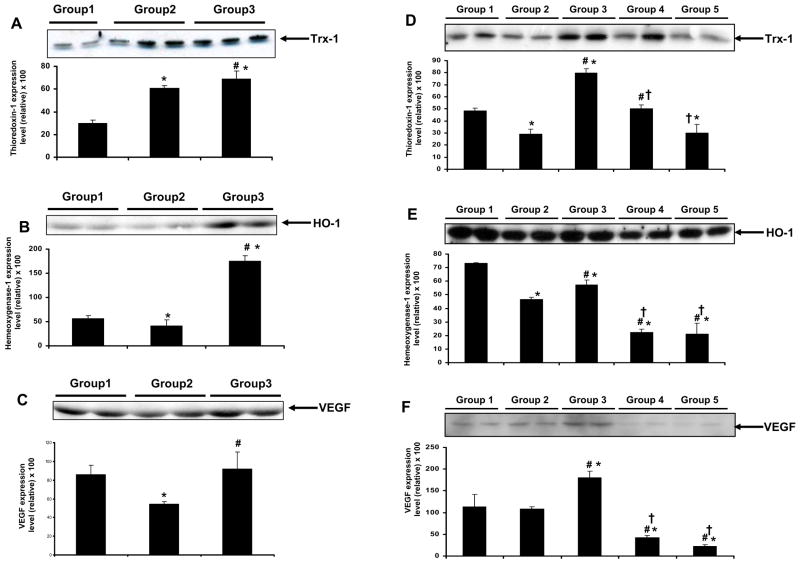
Effect of resveratrol on the protein expression of Trx-1, HO-1 & VEGF
Trx-1 expression was found to have increased at the baseline level and decreased after ischemia-reperfusion in the diabetic group. Dia + Rsvl group demonstrated increased protein expression of Trx-1 both at the baseline level (Figure 6A) and after ischemia-reperfusion (Figure 6D) compared to the other treated groups. The increase in Trx-1 at the baseline level in diabetic group might be a compensatory mechanism against the induced oxidative stress during diabetic condition. The reason behind decreased Trx-1 expression following ischemia/reperfusion was not known and needs further investigation. Trx-1 expression was significantly decreased in groups (Dia + Rsvl + L-NAME group & Dia + L-NAME group) as compared to Dia + Rsvl group.
Figure 6.
A–F. Representative Western blot showing the effect of resveratrol on the expression of Trx-1, HO-1 & VEGF in streptozotocin induced diabetic rat hearts at the baseline level (A–C) & rat hearts subjected to 30 mins of ischemia followed by 2 hours of reperfusion (D–F). The isolated hearts from rats of all the groups (n=6) were either taken at the baseline level (perfused for 3–4 minutes to avoid blood contamination) or subjected to 30 mins of global ischemia followed by 2 hrs of reperfusion. *p<0.05 groups 2, 3, 4 & 5 vs group 1; †p<0.05 groups 3, 4 & 5 vs group 2; #p<0.05 groups 3 vs group 4 & 5. Where Group 1-control rats, Group 2-diabetic rats, Group 3-diabetic rats treated with resveratrol, Group 4-diabetic rats treated with resveratrol + L-Name, Group 5 -diabetic rats treated with L-Name. GAPDH was used as the loading control.
HO-1 expression (Fig 6B) was found to be decreased in the diabetic group both at baseline and after ischemia reperfusion (Fig 6E). On treatment with resveratrol (Dia + Rsvl group) HO-1 expression was found to increase in the Dia + Rsvl group at the baseline level which was sustained even after ischemia reperfusion compared to the control. Dia + Rsvl + L-NAME & Dia + L-NAME groups have shown decreased expression of HO-1 compared to Dia + Rsvl group.
VEGF protein level was found to be increased both at the baseline level (Figure 6C) and following ischemia/reperfusion (Figure 6F) in Dia + Rsvl group when compared to diabetic and non-diabetic control groups. Decreased VEGF levels were observed in diabetic group when compared to non-diabetic control group. Following ischemia/reperfusion, L-NAME treated groups (Dia + Rsvl + L-NAME group & Dia + L-NAME group) have shown decreased expression of VEGF compared to Dia + Rsvl group. L-NAME was found to abolish the resveratrol mediated increase in VEGF expression supporting the notion that increased expression of VEGF is nitric oxide mediated. GAPDH was used as the loading control.
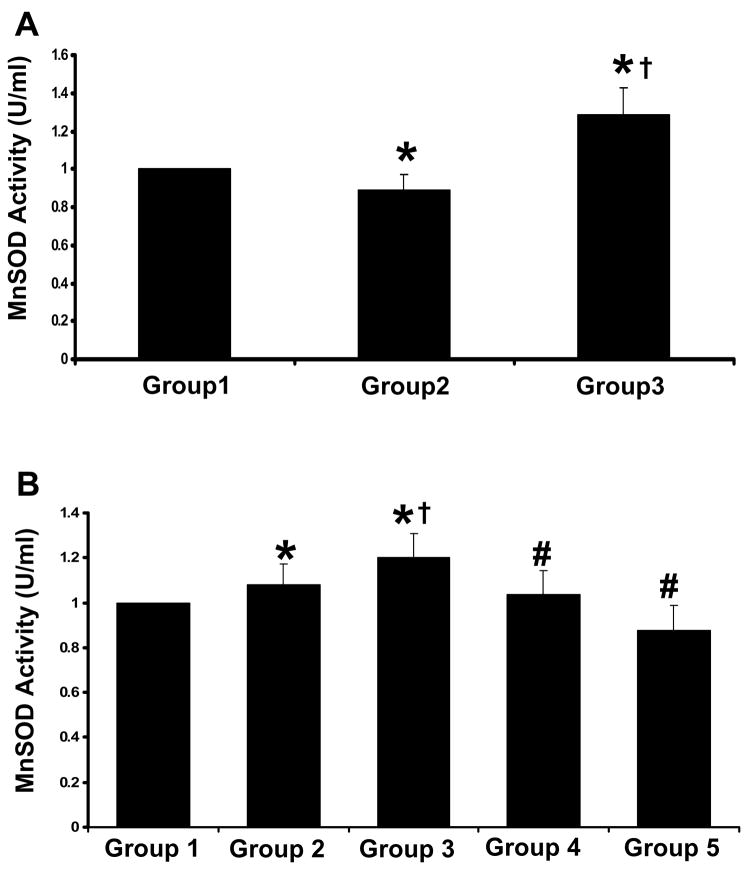
Effect of resveratrol on the Mn-SOD activity
The antioxidant status was measured by MnSOD activity assay. The activity of MnSOD was significantly lower in diabetic control at baseline and increased following ischemia/reperfusion injury when compared to non-diabetic control. The increase in MnSOD activity in diabetic group following ischemia/reperfusion might be a positive response to compensate the ischemic insult. Diabetic rats treated with resveratrol have shown marked increase in the MnSOD activity compared to diabetic and non-diabetic control both at baseline and after ischemia/reperfusion. L-NAME treated groups (Dia + Rsvl + L-NAME & Dia + L-NAME) have shown decreased MnSOD activity following ischemia/reperfusion when compared to resveratrol treated group. The increased MnSOD activity in resveratrol treated group might be due to the antioxidant property of resveratrol which was abolished on treatment with nitric oxide inhibitor.
DISCUSSION
A novel and important finding of the present study was that resveratrol treatment significantly reduced the blood glucose level in STZ-treated animals. The exact mechanism of resveratrol-induced amelioration of diabetic state remains unknown. However, several potential mechanisms are envisioned from other published data. First, increased production of NO in the skeletal muscle is known to decrease blood glucose level by increasing blood flow to the skeletal muscle and increasing glucose disposal (30). Secondly, reducing oxidative stress reverses insulin resistance by inhibiting redox-sensitive phosphorylation and down regulation of insulin receptor substrate-1 (31). It is known that hyperglycemia increases the production of reactive oxygen species (32). Therefore, resveratrol-induced decrease of blood glucose level can protect the heart from oxidative stress even in the absence of ischemia/reperfusion.
This study also suggests that resveratrol-induced attenuation of oxidative stress during ischemia/reperfusion is mediated by NO/Trx-1/HO-1/VEGF system and by a robust increase in potent antioxidant enzyme, Mn-SOD. This conclusion is supported by our various important findings in this study. Prior study has indicated that elevated super oxide (02−.) generation in the hyperglycemic state associated with diabetes, was attenuated with upregulation of HO-1 (33). In our study, treatment of diabetic rats with resveratrol induced Trx-1 and HO-1 significantly, which was abolished by nitric oxide synthase inhibitor. We believe that the action of resveratrol is largely or solely through the induction of nitric oxide (9, 34) or may be due to the induction of thioredoxin and HO-1 (14). Thioredoxin is a 13-Kd redox (reduction/oxidation)-active protein (35) containing a dithiol-active site, which is reversibly reduced by Trx reductase (36). Trx-1 has been suggested as a marker of oxidative stress in various diseases, as it is induced by oxidative stressor such as hydrogen peroxide, ultraviolet irradiation (37), and inflammation (38). Trx-1 shows a variety of biological activities, including scavenging of active oxygen radicals, and regulation of redox-sensitive molecules such as NFκB, AP-1 etc (37). Trx-1 which can scavenge free radicals has recently been found to protect cells from oxidative stress-induced apoptosis and to prevent STZ induced diabetes (39). More detailed study is needed to determine the clinical roles of Trx-1 in diabetes. In this context we assume thioredoxin has a potential for a new therapeutic approach to various chronic diseases including diabetes mellitus.
The cytoprotective effect of HO-1 and increased cell survival in vitro and in vivo have been previously described (40). However, little is known regarding the mechanism involved. HO-1 and its reaction products have been shown to have both antioxidative and anti-inflammatory properties, indicating a protective role of HO-1 in diabetes complications of myocardial infarction. Liu et al (41), using homozygous HO-1 null mice, demonstrated that an absence of HO-1 exacerbated myocardial injury, particularly in concert with diabetes. In the same study, the authors documented elevated cardiac HO-1 expression in response to ischemia/reperfusion, thus implicating a protective role of HO-1 in diabetic myocardial infarction. The other finding in the same study was that similar degree of hyperglycemia was observed after STZ injection in both HO-1+/+ and HO-1−/−, but diabetic HO-1−/− mice had more severe myocardial infarction which documents that upregulation of HO-1 protects against diabetic myocardial infarction. HO is the first and rate limiting enzyme in the heme breakdown to generate biliverdin, free ferrous iron and carbon monoxide (CO). Biliverdin is rapidly converted to bilirubin by biliverdin reductase. Presumably the protective effect of HO-1 depends upon these metabolites of heme catabolism along with HO-1 which appear to play vital roles in regulating important biological responses in diabetic myocardium. The production of CO and bilirubin via the HO system has been shown to be important protective factors in myocardial ischemia/reperfusion injury (42). However the exact protective mechanism of HO-1 by which this occur is still unclear. The finding that exogenous CO appears fully able to substitute for HO-1 on preserving ischemia reperfusion injury of transplanted hearts suggests that CO accounts in large measure for the protective effect of HO-1 (43). Again, the role of HO-1 in reduction of blood glucose level in our model is possible. Because the protective role of HO-1 in pancreatic islets that are exposed to high glucose concentration has been reported (44). In addition, in vitro HO-1 gene transfer has been shown to influence the viability and function of rat islets (45). Therefore our reports support the positive role played by resveratrol in glucose regulation and cardioprotection in diabetic myocardium by increasing HO-1 expression.
Again, following resveratrol therapy we found decreased blood glucose levels along with increased Mn-SOD activity significantly. Shen et al (46) demonstrated over expression of MnSOD improved respiration and normalized mass in diabetic mitochondria. MnSOD also protected the morphology of diabetic hearts and completely normalized contractility in diabetic cardiomyocytes. Elevating MnSOD activity in our present study may have provided extensive protection to diabetic mitochondria and supported overall protection to the diabetic heart.
The other major finding in the present study indicated significant induction of VEGF in resveratrol treated diabetic rats. We know that there is a strong relationship between NO and the regulation of blood vessel growth and development, especially that mediated by the actions of VEGF. Inhibitors of NOS and NO were found to suppress endogenous as well as VEGF induced angiogenesis (47). Furthermore, increased exposure to NO exogenously or via the genetic augmentation of NOS significantly potentiates the synthesis of VEGF in endothelial cells (48). A recent report suggested VEGF-induced expression of Bcl-2, which eventually functions to enhance the survival of endothelial cells in the toxic, oxygen-deficient environment (49). These reports point out that enhanced levels of VEGF may have some role in the inhibition of endothelial cell apoptosis. Another report demonstrated VEGF as a potent promoter of angiogenesis that upregulates the expression of the intracellular adhesion molecule-1 (ICAM-1) through a novel pathway that includes phosphatidylinositol -3 OH-Kinase (PI3-K) and AKT resulting in the migration of brain microvascular endothelial cells. It was found that in vitro VEGF treatment phosphorylated AKT in a PI3K-dependent manner (50). In our present study we documented significant activation of survival pathways as evidenced by robust phosphorylation of AKT in resveratrol treated diabetic myocardium following ischemia reperfusion which was abolished when L-NAME was given along with resveratrol therapy. The increased cardiomyocyte cell death found in diabetic myocardium was reduced with resveratrol treatment possibly due to nitric oxide dependent and VEGF mediated activation of survival pathway. This demonstrates a possible clinical role for resveratrol in protecting the vasculature from the damage associated with diabetes is mediated by nitric oxide mechanism.
Thus, our present study indicates NO mediated induction of Trx-1, HO-1 and VEGF by resveratrol in diabetic myocardium. In addition, resveratrol was also found to reduce blood glucose level, cardiomyocyte cell death in conjunction with increased Mn-SOD activity in diabetic rat myocardium which constitutes a novel mechanism for treating diabetic patients clinically in a similar fashion.
Figure 7. Effect of resveratrol on MnSOD activity.
(A) Baseline level - The hearts were isolated and perfused for 3–4 minutes to avoid blood contamination. (B) Ischemia/Reperfused - The isolated hearts from rats of all the five groups (n=6) were subjected to 30 mins of global ischemia followed by 2 hrs of reperfusion. *p<0.05 Groups 2, 3, 4 & 5 vs Group 1; †p<0.05 Groups 3, 4 & 5 vs Group 2; #p<0.05 Groups 4 & 5 vs Group 3. Where Group 1-control rats, Group 2-diabetic rats, Group 3-diabetic rats treated with resveratrol, Group 4 -diabetic rats treated with resveratrol + L-Name, Group 5-diabetic rats treated with 25 mg/kg b.wt of L-Name.
Acknowledgments
This study was supported by National Institutes of Health Grants HL 56803, HL 69910 and HL 85804.
List of abbreviations
- STZ
Streptozotocin
- Dia
Diabetic
- Rsvl
Resveratrol
- NO
Nitric oxide
- Trx-1
Thioredoxin-1
- HO-1
Heme oxygenase
- VEGF
Vascular Endothelial Growth Factor
- eNOS
endothelial Nitric oxide synthase
- MnSOD
Manganese Superoxide Dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobs J, Sena M, Fox N. The cost of hospitalization for the late complications of diabetes in the United States. Diabet Med. 1991;8:S23. doi: 10.1111/j.1464-5491.1991.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 2.Ray NF, Thamer M, Taylor T, Fehrenbach SN, Ratner R. Hospitalization and expenditures for the treatment of general medical conditions among the U.S. diabetic population in 1991. J Clin Endocrinol Metab. 1996;81:3671–79. doi: 10.1210/jcem.81.10.8855821. [DOI] [PubMed] [Google Scholar]
- 3.Abbud ZA, Shindler DM, Wilson AC, Kostis JB. Effect of diabetes mellitus on short- and long-term mortality rates of patients with acute myocardial infarction: a statewide study: Myocardial Infarction Data Acquisition System Study Group. Am Heart J. 1995;130:51–8. doi: 10.1016/0002-8703(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 4.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women: the Framingham Study. JAMA. 1988;260:3456–60. [PubMed] [Google Scholar]
- 5.Thompson EW. Quantitative analysis of myocardial structure in insulin-dependent diabetes mellitus: Effects of immediate and delayed insulin replacement. Proc Soc Exp Biol Med. 1994;205:294–305. doi: 10.3181/00379727-205-43710. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–8006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- 7.El-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI-3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–52. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- 8.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur J Endocrinol. 1998;138:619–20. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Kaga S, Zhan L, Bagchi D, Bertelli A, Maulik N. Beneficial effect of pharmacological intervention by resveratrol induces myocardial angiogenesis by upregulating VEGF and Tyrosine Kinase Receptor, Flk-1/KDR. Cell Biochem Biophys. 2006;44:43–9. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 10.Jager U, Nguyen-Duong H. Relaxant effect of trans-resveratrol on isolated porcine coronary arteries. Arzneimittelforschung. 1999;49:207–11. doi: 10.1055/s-0031-1300403. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AA, Panichi V, Filippi C, Bertelli A. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001;37:262–70. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;282:H1988–95. doi: 10.1152/ajpheart.01012.2001. [DOI] [PubMed] [Google Scholar]
- 13.Naderali EK, Doyle PJ, Williams G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin Sci (Lond) 2002;98:537–43. [PubMed] [Google Scholar]
- 14.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol Enhances Neovascularization in the infracted rat myocardium through the induction of Thioredoxin-1, Hemeoxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico M, Marfella R, Nappo F, Di Filippo C, De Angelis L, Berrino L, Rossi F, Giugliano D. High glucose induces ventricular instability and increases vasomotor tone in rats. Diabetologia. 2001;44:464–70. doi: 10.1007/s001250051644. [DOI] [PubMed] [Google Scholar]
- 16.Arner ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–09. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 17.Kietzmann T, Samoylenko A, Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J Biol Chem. 2003;278:17927–36. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 18.Terry CM, Clikeman JA, Hoidal JR, Callahan KS. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am J Physiol. 1998;274:H883–91. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 19.Andre M, Felley-Bosco E. Heme oxygenase-1 induction by endogenous nitric oxide: influence of intracellular glutathione. FEBS Lett. 2003;546:223–7. doi: 10.1016/s0014-5793(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 20.Maines MD. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB. 1988;2:2557–68. [PubMed] [Google Scholar]
- 21.Otterbein LE, Bach FH, Alam J, Soares M, Lu HT, Wysk M, Davis RJ, Flavell RA, Choi AMK. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 22.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signaling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–394. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 23.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, DellAcqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long term myocardial protection using adeno-associated virus-mediated delivery of heme-oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 24.Maulik N, Sasaki H, Addya S, Das DK. Regulation of cardiomyocyte apoptosis by redox-sensitive transcription factors. FEBS Letters. 2000;485:7–12. doi: 10.1016/s0014-5793(00)02174-8. [DOI] [PubMed] [Google Scholar]
- 25.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Kisin E, Kagan VE, Maulik N, Das DK. Hypoxic preconditioning preserves antioxidant reserve in the working rat heart. Cardiovasc Res. 1995;29:133–40. [PubMed] [Google Scholar]
- 26.Sasaki H, Galang N, Maulik N. Redox regulation of NFkB and AP-1 in ischemic reperfused heart. Antioxi Redox Signaling. 1999;1:317–24. doi: 10.1089/ars.1999.1.3-317. [DOI] [PubMed] [Google Scholar]
- 27.Thirunavukkarasu M, Penumathsa S, Juhasz B, Zhan L, Cordis G, Altaf E, Bagchi M, Bagchi D, Maulik N. Niacin bound chromium enhances myocardial protection from ischemia reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H820–6. doi: 10.1152/ajpheart.00134.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 29.Maulik N, Yoshida T, Zu YL, Sato M, Banerjee A, Das DK. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. Am J Physiol. 1998;275:H1857–64. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- 30.Baron AD, Clark MG. Role of blood flow in the regulation of muscle glucose uptake. Annu Rev Nutr. 1997;17:487–99. doi: 10.1146/annurev.nutr.17.1.487. [DOI] [PubMed] [Google Scholar]
- 31.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 32.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 33.Abraham NG, Rezzani R, Rodella L, Kruger A, Taller D, Li Volti G, Goodman AI, Kappas A. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol. 2004;287:H2468–77. doi: 10.1152/ajpheart.01187.2003. [DOI] [PubMed] [Google Scholar]
- 34.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–71. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 36.Luthman M, Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 1982;21(26):6628–33. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annual Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 38.Bertini R, Howard OM, Dong HF, Oppenhein JJ, Bizzarri C, Sergi R, et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189(11):1783–9. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J. Pancreatic beta cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med. 1998;188:1445–51. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan S, Kaminski PM, Yang L, Morita T, Inaba M, Ikehara s, Goodman AI, Wolin MS, Abraham NG. Heme oxygenase-1 prevents superoxide anion-associated endothelial cell sloughing in diabetic rats. Biochem Biophys Res Commun. 2004;315(2):509–16. doi: 10.1016/j.bbrc.2004.01.086. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54(3):778–84. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 42.Stocker R, yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 43.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH. Soares P.M., Heme oxygenase-1 derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 44.Won KC, Moon JS, Eun MJ, Yoon JS, Chun KA, Cho IH, Kim YW, Lee HW. A protective role for Heme oxygenase -1 in INS-1 cells and rat islets that are exposed to high glucose conditions. J Korean Med Sci. 2006;21:418–424. doi: 10.3346/jkms.2006.21.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen XB, Li YX, Jiao Y, Dong WP, Li G, Chen J, Tan JM. Influence of heme oxygenase-1 gene transfer on the viability and function of rat islets in in vitro culture. World J Gastroenterol. 2007;21:1053–1059. doi: 10.3748/wjg.v13.i7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55(3):798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 47.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94(5):2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jozkowicz A, Cooke JP, Guevara I, Huk I, Funovics P, Pachinger O. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001;51(4):773–83. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 49.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]