Table 1. ATPase Activity and Motility of Kinesin Derivatives.
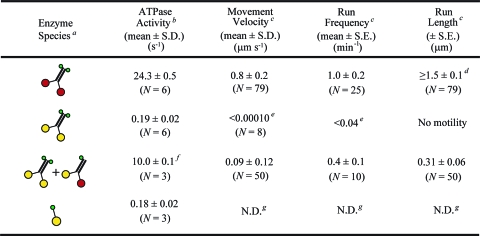 |
Schematic diagrams of enzyme species present, using the same symbols as in Figure 1.
Microtubule-stimulated ATPase specific activity per dimer (rows 1−3) or per monomer (row 4) in 1 mM ATP, 1.2 mg/mL microtubules, 25 °C.
In single-molecule bead movement assay at 1:1 enzyme dimer:bead mole ratio, 1 mM ATP, 22−23 °C.
Data set included some runs where motor ran off the microtubule end.
No motility detected. Value given is the detection limit.
Implies that heterodimer specific activity is ∼15 s−1, since the preparation is estimated by gel densitometry to contain ∼35% mutant homodimers, which have negligible activity.
Not determined.
